Abstract
Moringa oleifera Lam. (Sohanjna, Drumstick tree, Horseradish tree; family, Moringaceae) is a well-known plant, native to Pakistan, Bangladesh, India, Sri Lanka, Philippines and Africa. It is known as miracle tree as well as mother’s best friend. Almost every part of plant (i.e., leaves, seeds, bark, root and wood) is a rich source of vitamins, minerals (calcium, magnesium and phosphorus), carotenes and folic acid. Various parts of M. oleifera such as pods, roots and bark have been reported for kidney stone disease; however, no such data is available to show the antiurolithiatic potential of seeds. The aim of the study was to validate the antiurolithiatic potential of Moringa oleifera Lam. seeds against ethylene glycol-induced urolithiasis.
Aqueous alcoholic extract of Moringa oleifera Lam. seeds (MoS.Cr) was prepared and phytochemical screening was carried out to assess the medicinally important phytoconstituents. To evaluate the antiurolithic potential, Wistar albino male rats were divided into different groups. All the groups except normal control group received lithogenic treatment (1% ammonium chloride +0.75% EG) for 21 days. Normal control and intoxicated groups received D. W (5ml/kg; p.o.), treatment groups received MoS. Cr at the doses of 100, 300 and 500mg/kg; p.o. while the standard group received cystone (500mg/kg; p.o.) for the next 14 days. After 21 and 35 days, animals were placed individually in metabolic cages and urine samples were analyzed for different parameters, i.e., crystal count, urine pH, urine volume, uric acid, calcium, magnesium, phosphorus and total protein levels. After the completion of study (at 35th day), blood samples were collected via retro-orbital technique, serum was separated and analyzed to determine serum creatinine and blood urea nitrogen levels. Histological studies were performed to evaluate the protective effects of MoS.Cr against ethylene glycolinduced urolithiasis. MoS.Cr was found safe upto the dose of 10 g/kg when tested for toxicity. Phytochemical screening revealed the presence of alkaloids, phenols, flavonoids, tannins, saponins, glycosides and coumarins. MoS.Cr showed dose-dependent effects and restored the biochemical parameters towards normal. The results conclude that the seeds of M. oleifera possess stone inhibiting potential mediated possibly due to antioxidant potential, urine alkalinizing and diuretic effects, thus providing scientific background for its use against urolithiasis.
Keywords: Moringa oleifera; Urolithiasis; Crystal count; Ethylene glycol; Ammonium chloride
Abbreviations: MoS.Cr: Methanolic Aqueous Extract of Moringa Oleifera Seeds; AC: Ammonium Chloride; EG: Ethylene Glycol; BUN: Blood Urea Nitrogen; CaOx: Calcium Oxalate Crystals
Introduction
Urolithiasis is a debilitating condition in which one or more calculi are formed in any part of the urinary system and the stones are classified according to their location in the urinary system, i.e., kidney stones, bladder stones, ureteral and urethral calculi. Kidney stones are classified on the basis of composition, i.e., calcium oxalate, calcium phosphate, uric acid, struvite and cystine stones. The two main types of calcium oxalate (CaOx) crystals include calcium oxalate monohydrate and calcium oxalate dihydrate. There are four main steps of stone formation, i.e., supersaturation, nucleation, aggregation and crystal growth. Supersaturation occurs when there is less amount of water and high concentration of solutes (calcium salts). Most of the renal stones are small and can easily pass out through the urinary tract without any irritation and pain. While some stones are larger and cannot pass through the urinary system and get attached to the renal epithelial cells causing damage through oxidative stress [1].
Urolithiasis is a common and serious problem throughout the world affecting approximately 12% of the population. In Asian countries, it is a recurrent life-threatening disorder and the recurrence rate is upto 50% during 5-10 years. In Pakistan, the prevalence rate of urolithiasis is about 16% [2]. For urolithiasis, men are at higher risk than women (3:1) which can be associated with many predisposing factors like levels of testosterone and nutritional differences. Diet containing higher amount of animal protein can enhance the risk of urolithiasis. High water intake can reduce the risk by causing dilution of urine and reducing the stay time of free stone particles in urine. The incidence of kidney stone formation is common in summer due to sweating which causes precipitation of urinary constituents. Low urinary levels of magnesium and citrate while the high levels of calcium, oxalate and uric acid can cause a higher risk of urolithiasis [3].
According to WHO, about 75% of the world’s population depends upon herbal remedies due to their less adverse effects. Herbal medicines are also known as natural medicines. Plants are important and the cheapest source of medicinal constituents that play significant role to treat various diseases. Scientific studies have discovered many plant-derived medicines through the study of folk use of herbal plants against several diseases [4]. Moringa oleifera Lam. commonly known as Sohanjna belongs to family Moringaceae and it is reported to have antimicrobial, antifungal, antibacterial, anti-inflammatory, antioxidant, antitumor, antifertility, hepatoprotective, antihypertensive, hypocholesterolemic, antiulcer, antipyretic, antidiabetic, anticonvulsant and anti-allergic activities [5]. Various parts of the plant such as dried root bark, dried root wood, fresh bark and fresh pods have already been reported against urolithiasis. However, no data is available regarding the usefulness of seeds against urolithiasis. The diversity and multiplicity of actions as well as the usefulness of all the parts of plant as medicinal remedies compelled the researchers to evaluate the potential of Moringa oleifera Lam. seeds against ethylene-glycol induced urolithiasis in rats.
Research Methodology
a. Materials and Equipment
All the chemicals and kits used in research work were of the analytical grade. To estimate the biochemical parameters (Creatinine, Phosphorus, Magnesium, Calcium, Uric acid, Total protein and Urea), assay kits of Human Diagnostic, Germany, were used. Chemicals such as Ammonium chloride (Lahore Pharma, Lahore), Ethylene glycol (Merck, Germany), Cystone (Himalaya, India and Batch no. 112000673), Xylazine (My Lab, Bahawalpur, Pakistan), Ketamine (Global Pharmaceutical, Islamabad, Pakistan) and Formalin (Riedel-de Haen, Germany) were used in the study. Equipment used during the research work were Digital weighing balance (Shimadzu, AY62, Japan), Rotary evaporator (Heidolph, Laborota 4000-efficient, Germany), Metabolic cages (Tecniplast, Italy), pH meter (inoLAB, pH720, Germany), Hemocytometer (Marienfeld, Germany) and UV-visible spectrophotometer (Irmeco, U2020, Germany).
b. Collection of Plant Material
Dried seeds of Moringa oleifera Lam. were procured from the Baghdad-ul-Jadeed campus, the Islamia University Bahawalpur. The plant was authenticated by botanist, Mr. Abdul Hameed, department of Life Sciences, IUB and sample of dried seeds deposited in the herbarium of Pharmacology research laboratory, department of Pharmacology, faculty of Pharmacy, the Islamia University of Bahawalpur, Pakistan. Voucher numbers for seeds (MO-SD-10-20-171) was issued for future reference.
c. Preparation of the Crude Extract of Moringa oleifera Lam. seeds
1.5kg seeds of Moringa oleifera Lam. were crushed into coarse powder and soaked in 70% aqueous methanol. After three days, the soaked plant was filtered through a muslin cloth and then with Whatman grade 1 filter paper. Soaking and filtration were repeated twice. After the third filtration, residues were discarded and the filtrate was subjected to rotary evaporator under the reduced pressure to prepare a concentrated semi solid paste and then dried in a hot air oven. Then, the semisolid form of Moringa oleifera Lam. seeds extract (MoS.Cr) was weighed, labelled and stored in freezer for future use.
d. Qualitative Phytochemical Screening
Phytochemical analysis of MoS.Cr was carried out to assess the presence of different secondary metabolites like alkaloids, carbohydrates, flavonoids, glycosides, coumarins, phenols, phlobatannins, proteins and amino acids, quinones, resins, saponins, tannins and terpenes.
e. Experimental Animals
Adult male Wistar albino rats (150-270g) and Swiss mice (20- 30g) were used in the study. Animals were kept under environmental conditions, i.e., temperature (23±2°C), 12 h light/dark cycle and fed with standard diet and water ad libitum. All animals were housed in the animal house of Pharmacology research laboratory, department of Pharmacology, faculty of Pharmacy, the Islamia University of Bahawalpur. The experimental procedures and protocols were approved by the Pharmacy Animal Ethics Committee (PAEC) under reference number PAEC/2020/27.
f. Antiurolithic Potential of MoS.Cr
To investigate the antiurolithic potential of MoS.Cr, calcium oxalate (CaOx) urinary crystals were induced in albino rats by using 1% ammonium chloride (AC) and 0.75% ethylene glycol (EG) for the first 5 days and then EG alone for the next 16 days in drinking water.
g. Animal Model of Urolithiasis
The effects of MoS.Cr against urolithiasis were studied by dividing the animals into different groups of matched body weight. One group was considered as normal control which received tap water while other groups received lithogenic treatment for the first 21 days. After 21 days, lithogenic treatment was withdrawn and the respective treatments were administered for the next 14 days. Normal control group and intoxicated group received distilled water (5ml/kg p.o.) once per 24hr. Standard group received cystone (500mg/kg p.o.) once per 24hr. While remaining groups received different doses of MoS.Cr, i.e. 100, 300 and 500mg/kg p.o. once per 24 hours respectively.
h. Urine Collection and Analysis
All the animals were kept in metabolic cages at the end of day 21 and day 35 for collection of urine. Fresh urine samples were collected in the morning. For urinary crystal count, 1ml of each urine sample was taken and centrifuged at 3000rpm for 5 minutes. 950μl of supernatant was discarded and remaining portion was transferred to a Neubauer chamber. The types and number of crystals were determined using a light microscope as described previously [6]. After 24 hours, Urine samples were collected. Urinary pH and urinary volume per 24hrs were determined. Urinary levels of uric acid, phosphorus, magnesium, calcium and total protein were determined by using commercially available kits.
i. Serum Analysis
The animals were anesthetized with Ketamine/Xylazine (10:1) at the end of the day 35. Blood was collected through retro-orbital method and allowed to clot for 15 minutes. Then, centrifuged at 4000rpm for 15 minutes and serum was separated. Serum analysis was done for the determination of different biochemical parameters, i.e., creatinine and blood urea nitrogen.
j. Kidney Histology
At the end of study, animals were sacrificed. One representative kidney from each group was removed, preserved in 10% formalin and then analyzed histologically.
k. Acute Toxicity Assay
Acute toxicity test was carried out to assess the safety of MoS.Cr on Swiss albino mice of either sex weighing (18-30g) as described previously [7]. Animals were randomly divided into different groups of five mice each. Normal control received distilled water (10ml/ kg p.o). Different doses of MoS.Cr were given; i.e. 0.3, 1, 3, 5 and 10g/kg; p.o. to investigate the toxicity of the extract. The mice were observed critically for 2hrs and then at the interval of 30 minutes for next 6hr for any type of behavioral changes. The animals were observed for the next 48hrs.
Results
l. Phytochemical Screening of MoS.Cr
The aqueous alcoholic extract (MoS.Cr) was found to be rich in alkaloids, saponins, flavonoids, phenolic contents, glycosides, tannins and coumarins.
m. Effects of MoS.Cr on Urine Parameters
In present study, urine parameters, i.e., crystal count, urine volume and urine pH were determined at 21st and 35th day. While uric acid, calcium, magnesium, phosphorus and total protein levels were monitored at 35th day. The effects of different doses of MoS.Cr; i.e. 100, 300 and 500mg/kg, on crystal count, urine volume, urine pH, uric acid, calcium, magnesium, phosphorus and total protein are shown in Figure 1. Only few crystals were shown in 3h morning urine sample of normal control group. While lithogenic treatment showed that intoxicated rats excreted abundant and enormous crystals. MoS.Cr showed highly significant and dose-dependent effects, i.e., decreased crystals and the results of MoS.Cr at the dose of 500mg/kg were almost comparable to the results of cystone (Figure 1A).
Lithogenic treatment induced urolithiasis and caused significant increase in urine volume, uric acid, phosphorus and total protein levels. While the urine pH, calcium and magnesium levels were decreased after the intoxication. MoS.Cr, at the doses of 100 and 300mg/kg, did not show any significant reduction in urine volume, but at the dose of 500mg/kg, showed highly significant results (p<0.001) and reduced the urine volume (Figure 1B). Lithogenic treatment consists of ethylene glycol and ammonium chloride (AC), AC decreased the urine pH and enhanced the deposition of CaOx crystals in intoxicated groups. MoS.Cr showed the highly significant results and increased the urine pH in dose-dependent manner (Figure 1C). Calcium and magnesium (stone inhibitor) levels were decreased in lithogenic rats (Figure 1E and 1F, respectively). While Uric acid, phosphorus and total protein levels were increased after the lithogenic treatment (Figure 1D, 1G and 1H, respectively). MoS.Cr, at the doses of 100, 300 and 500mg/kg p.o. significantly reversed and restored these urinary changes towards normal and the results of MoS.Cr were almost comparable to the results of cystone (500mg/kg), the standard drug.
Figure 1: The effects of MoS.Cr and cystone on urine parameters (A) Crystal count, (B) Urine volume, (C) Urine pH, (D) Urine Uric acid, (E) Urine Calcium, (F) Urine Magnesium, (G) Urine Phosphorus, (H) Urine Total protein.
Mean ± SEM; n=6; ns: p>0.05; *: p<0.05; **: p<0.01; ***&###: p<0.001
(*: Comparison within the groups, i.e., 21st and 35th day and #: comparison of intoxicated group with normal groups at 21st day; One-way ANOVA followed by bonferroni’s post hoc test).
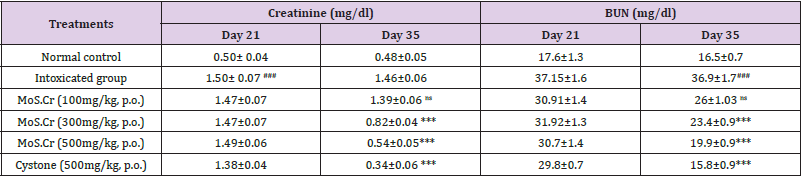
n. Effects of MoS.Cr on Serum Parameters
Lithogenic treatment caused highly significant increase (p<0.001) in serum creatinine and BUN levels. MoS.Cr showed highly significant results and reduced the creatinine and BUN levels (Table 1).
Table 1: Effects of MoS.Cr and cystone, the standard drug, on serum parameters in curative model of urolithiasis.
Mean±SEM; n=6; ns: p>0.05; *: p<0.05; **: p<0.01; ***&###: p<0.001
(*: Comparison within the groups, i.e. 21st and 35th day and #: comparison of intoxicated group with normal
o. Effects of MoS.Cr on Histological Parameters
Intoxicated group showed pronounced epithelial cell damage, enlarged interstitial spaces, and disarrangements after 21 days of lithogenic treatment. MoS.Cr showed dos- dependent effects at the doses of 100, 300 and 500mg/kg and improved the integrity of renal tubular epithelium (Figure 2).
Figure 2: Histological sections of kidney; (a) Control (D. W 5ml/kg) (b) Intoxicated (AC/EG) (c) Standard (Cystone; 500mg/ kg) (d) MoS.Cr; 100mg/kg (e) MoS.Cr; 300mg/kg (f) MoS.Cr; 500mg/kg ( : Enlarged interstitial spaces of renal tubular epithelium).
p. Acute Toxicity Assay
Acute toxicity of MoS.Cr was performed according to OECD guidelines and found to be safe upto 10g/kg. No sign of toxicity and behavioral changes such as alertness, convulsions, grooming, hyperactivity, salivation, urination, lacrimation, pain response, touch response, corneal reflex, writhing reflex, gripping strength, righting reflex and skin color were observed. No mortality was noted after 48hrs.
Discussion
Moringa oleifera Lam. seed extract was studied to investigate its antiurolithiatic potential against ethylene glycol-induced urolithiasis. Urinary supersaturation is considered as one of the major risk factors of urolithiasis. Stone’s formation in ethylene glycol treated animals is due to the hyperoxaluria that increases urinary oxalate concentration which forms the complex with calcium and stone formation occurs [8]. MoS.Cr decreased (p<0.001) the crystals count in dose-dependent manner. Urinary stones interfere with functions of kidney, decrease the reabsorption of water and enhance the urine volume. MoS.Cr significantly reduced the ethylene-glycol induced polyuria, but the urine volumes of treatments group were even greater than the respective normal control group which could be attributed to the natural diuretic activity of the plant and its constituents [9]. Acidic urinary pH has significant impact on the deposition of urinary calcium oxalate crystals. Whereas alkaline pH favors the deposition of calcium phosphate crystals. Ammonium chloride decreases the urinary pH to acidic value and decreases the solubility of calcium oxalate crystals. Therefore, analysis of urine showed the excessive levels of calcium oxalate crystals after 21 days of lithogenic treatment [10].
Uric acid is formed from the metabolism of purine rich diet such as meat, beans, green peas, cheese and oats etc. Uric acid acts as nucleator and accelerate the process of crystals formation. It also precipitates the calcium oxalate crystals by decreasing its solubility [11]. Lithogenic treatment caused significant increase in urinary uric acid levels, which were decreased significantly after the treatment with MoS.Cr. Normal urine consists of organic and inorganic inhibitors. Crystallization occurs when there is an imbalance between stone promoters and stone inhibitors. Magnesium and citrate are considered as stone inhibitors. Magnesium containing drugs and diet have significant impact on magnesium levels, e.g., potassium magnesium citrate, can prevent the recurrence of stones in urine. Magnesium forms the complex with oxalate and reduces the precipitation of calcium oxalate crystals. Ethylene glycol caused severe reduction in urinary magnesium levels which were restored to a significant value with the treatment of MoS.Cr. In crystalluria, there is a negative correlation between the excretion of oxalate and calcium. Lithogenic treatment increased the urinary oxalate levels but decreased calcium levels. For kidney stones, ethylene glycolinduced hyperoxaluria is a major risk factor and excessive levels of oxalate form the complex with calcium and decrease the solubility of calcium oxalate complex in urine. MoS.Cr was found to improve the levels of calcium in urine in dose- dependent manner [10].
An increase in urinary phosphorus levels along with high levels of oxalate in ethylene glycol- induced urolithiasis favors the formation of stones by forming the calcium phosphate crystals. Calcium phosphate crystals cause precipitation and deposition of calcium oxalate crystals. Raised protein level in urine is known as proteinuria. The present results showed increased levels of urinary protein after 21 days of intoxication. Proteinuria indicates the dysfunction in the proximal tubule of kidney. Proteinuria causes supersaturation of urinary constituents and initiates the crystals formation process. MoS.Cr restored the phosphorus and total protein level to a significant extent [11]. Creatinine is a nitrogenous waste product which is formed by the natural breakdown of muscle tissues and then secreted from the blood by kidney. Levels of serum creatinine and urea indicate the efficiency of kidney. In urolithiasis, glomerular filtration rate (GFR) decreases due to renal damage and the level of nitrogenous waste product increases. Concentration of creatinine in serum was increased due to the consumption of ethylene glycol. MoS.Cr showed significant restoration of increased levels of creatinine towards normal in increasing doses [12].
Conclusions of the Study
The present study demonstrates the antiurolithiatic potential of Moringa oleifera Lam. seeds against ethylene glycol-induced urolithiasis. The phytochemical screening revealed the presence of alkaloids, phenols, flavonoids, glycosides, carbohydrates, tannins and quinones in the extract. MoS.Cr showed significant restoration of the biochemical parameters (crystals count, urine volume, urine pH, uric acid, calcium, magnesium, phosphorus, total protein, serum creatinine and BUN) towards normal dose-dependently (100, 300 and 500 mg/kg). Acute toxicity test showed that MoS.Cr was safe upto the dose of 10g/kg. Thus the study suggests that the seeds of M.oleifera possess antilithiatic potential against calcium oxalate crystals which may be due to the presence of crystals inhibitory, urine alkalinizing, diuretic and antioxidant effects, hence providing the scientific ground for the folkloric use of M. oleifera against urolithiasis.
Acknowledgment
Authors acknowledge the contribution of Prof. Dr. Shazia Anjum (Director, Cholistan Institute of Desert Studies, IUB) and HEC project of National Research Program for Universities (NRPU No. 6300) for supporting and providing the basic material for this research.
Conflict of Interest
The authors declare no conflict of interest.
Authors Contribution
Hina Ali planned the study and performed the experiments under the supervision of Prof. Dr. Qaiser Jabeen. Ayesha Nadeem contributed to drafting of manuscript and Mariya Anwaar contributed in the preparation of extract. All authors approved the manuscript after complete reading.
References
- Kant R, Singh TG, Singh S (2020) Mechanistic approach to herbal formulations used for urolithiasis treatment. Obesity Medicine 19.
- Goyal PK, Verma SK, Sharma AK (2017) Antilithiatic potential of Vernonia cinerea against calcium oxalate calculi in experimental rats. The Journal of Phytopharmacology 6(2): 149-155.
- Sellaturay S, Fry C (2008) The metabolic basis for urolithiasis. Surgery (Oxford) 26(4): 136-140.
- Gilani AH (2005) Trends in ethnopharmacology. Journal of Ethnopharmacology 100(1-2): 43-49.
- Paikra BK (2017) Phytochemistry and pharmacology of Moringa oleifera Lam. Journal of Pharmacopuncture 20(3).
- Khan A, Bashir S, Khan SR, Gilani AH (2011) Antiurolithic activity of Origanum vulgare is mediated through multiple pathways. BMC Complementary and Alternative Medicine 11(1): 1-16.
- Jabeen Q, Bashir S, Lyoussi B, Gilani AH (2009) Coriander fruit exhibits gut modulatory, blood pressure lowering and diuretic activities. Journal of Ethnopharmacology 122(1): 123-130.
- Divakar K, Pawar AT, Chandrasekhar SB, Dighe SB, Divakar G (2010) Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food and Chemical Toxicology 48(4): 1013-1018.
- Bashir S, Gilani AH (2009) Antiurolithic effect of Bergenia ligulata rhizome: an explanation of the underlying mechanisms. Journal of Ethnopharmacology 122(1): 106-116.
- Bashir S, Gilani AH (2011) Antiurolithic effect of berberine is mediated through multiple pathways. European Journal of Pharmacology 651(1-3): 168-175.
- Rao GMM, Rao CV, Pushpangadan P, Shirwaikar A (2006) Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia Linn. Journal of Ethnopharmacology 103(3): 484-490.
- Mosquera DMG, Ortega YH, Quero PC, Martínez RS, Pieters L (2020) Antiurolithiatic activity of Boldoa purpurascens aqueous extract: An in vitro and in vivo study. Journal of Ethnopharmacology 253: 112691.

 Research Article
Research Article