Abstract
Emerging contaminants such as pharmaceuticals, personal care products, surfactants, industrial additives, plasticizers, pesticides, and several chemical compounds; affect the health of humans and animal species even when they are present in low concentrations. The objective of this work was to assess the effect of current intensity (CI), electrolysis time (ET), electrolyte concentration (EL), and electrolyte type (ELT) on the removal of Carbamazepine (CBZ) and Ibuprofen (IBP) from water. Electrooxidation process was investigated using Ti/PbO2(anode) and Ti(cathode) cylindrical electrodes in the presence of sodium sulfate (NaSO4) and ammonium sulfate ((NH4)2SO4) as electrolyte. The initial concentration in all treatments was 5 mg/L for each pollutant. A cylindrical reactor with concentric electrodes was used. The electrodes were distributed from center to periphery (cathode-anode-cathode). A Box-Behnken design was carried out to evaluate CBZ and IBP removal with a total of twenty different experimental conditions. Pollutant’s removal was affected for two of four variables studied (CI and ET), showing significant difference. Optimum results were obtained with CBZ reaching a removal of 92.92 %, in the case of IBP only 65.85 % of removal was found. These results were obtained with 1.26 Amperes of CI, 80 minutes of ET, 3 g/L of EL and (NH4)2SO4 as ELT. It was probed that the ET and CI are variables responsible for the pollutant’s removal. The effect of the ELT is only significant in the removal of ibuprofen. The EL showed no significant difference between treatments.
Keywords: Carbamazepine; Ibuprofen; Pollutant; Treatment; Water/p>
Introduction
It is common to speak about emerging contaminants such as pharmaceuticals, personal care products, surfactants, industrial additives, plasticizers, pesticides and several chemical compounds. Although these compounds are usually found in concentrations of mg/L or ng/L [1]. They cause significant biological effects such as endocrine system disruption, hormonal blocking functions [2]. Carbamazepine is an important pharmaceutical drug due to its use in clinics in the treatment of epilepsy and neuropathic pain, its high recalcitrance, and its ecotoxicological potential [3]. Ibuprofen is a non-steroidal anti-inflammatory drug that has been widely used to treat pain and inflammation in rheumatic disease and other musculoskeletal disorders [4]. Some wastewaters have extremely high chemical oxygen demand (COD), on average about 2000 mg/L, displaying a strong odor and dark color. Therefore, proper treatment of these effluents is essential before being discharged into water bodies [5]. The main methods for removing contaminants from wastewaters are physical, chemical and biological processes that occur in conventional processing methods. However, these methods are not efficient to remove micropollutants. Electrochemical technologies have reached a promising stage of development and they also can be used effectively to remove these compounds [6].
Furthermore, [7] reported that in recent years, the electrocoagulation has been successfully used to treat a variety of industrial wastewaters [8]. mentioned that electrocoagulation process has been applied to treat a variety of dye effluents. Electrooxidation is a process that degrades pollutants without forming other waste contaminants such as blood clots or flocs. Electrooxidation requires a supporting electrolyte to enhance electrical conductivity. Recent work has shown that sodium sulfate is the best supporting electrolyte for the electrochemical process considering economy, efficiency, and environmental aspects [2]. Box-Behnken have been previously using for determining the optimum operating conditions of COD [9]. This design includes uniformly distributed points in the space of encoded variables. One advantage is the ability to explore the entire experimental region and the usefulness of response interpolation. Besides the matrix allows the description of a region around an optimal response. Because of the importance of emerging contaminants removal from aquatic systems, the target for this research was to assess the effect of current intensity, electrolysis time, electrolyte concentration, and electrolyte type on the removal of Carbamazepine and Ibuprofen from water.
Materials and Methods
Synthetic Solution
CBZ and IBP analytical grade were used, to prepare a standard calibration curve. A stock solution (10 mg/L of each pollutant) was prepared using distilled water. Pharmaceuticals were solubilized by stirring for 24 hours at room temperature. This solution was kept at 4°C. Subsequently, 500 mL of stock solution were diluted with distilled water until reach a volume of litter. Then it was kept under stirring for 10 minutes. The resulting mixture with 5 mg/L of each pollutant was used to carry out all the experiments.
Experimental Unit
A cylindrical electrooxidation reactor was manufactured using acrylic material with 10 cm of radius, 20 cm of height, and 1500 mL of total volume. The trading volume was 1100 mL. Two circular titanium mesh electrodes were used as cathode and a circular titanium mesh electrode lead dioxide coated was used as anode. All electrodes were placed in concentric arrangement with interposed the center to the periphery (cathode-anode-cathode) to maximize conductivity efficiency. The spacing between electrodes was 1 cm.
Studied Variables
The effect of four variables, CI, ET, EL and ELT over carbamazepine (CBZR) and ibuprofen removal (IBFR), and Energy consumption (EC). A calibration curve of known concentration (0.1 to 5 mg/L) versus relative absorbance was used to calculate the concentration of the pharmaceuticals and to estimate removal efficiency. A total of 34 different experiments or treatments resulted using Box- Behnken design. Samples of each experiment were obtained at the beginning and at the end of each treatment. Carbamazepine and Ibuprofen concentration in the solution was determined by UV spectrophotometry at 285 and 210 nm respectively. Removal efficiency was calculated according to the following equation:
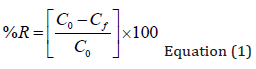
%R : Percentage of atrazine removed.
C0: Initial concentration of atrazine.
Cf: Final concentration of atrazine
Results and Discussion
Results were analyzed with Design Expert 7 software. Optimal values for the studied variables, maximizing pollutants removal and minimizing energy consumption, were obtained, which are presented in Table 1. In addition, significant effect of the variables is demonstrated for the ANOVA analysis (F < 0.05).
Conclusion
CBZ and IBP removal is affected mainly by two variables, CI, and ET, showing significant difference. Optimum remotion for CBZ was 92.92 %, while for IBP only 65.85 % of removal was found. These results were achieved with 1.26 Amperes of CI, 80 minutes of ET, 3 g/L of EL and (NH4)2SO4 as ELT. It was probed that the ET and CI are the main variables responsible for the pollutant’s removal. The effect of the ELT is only significant in the case of ibuprofen removal. The EL showed no significant difference between treatments. This process seems to be a promising technology that could be used as tertiary treatment to remove emerging contaminants from the water.
References
- Lapworth DJ, Baran N, Stuart ME, Ward RS (2012) Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ Pollut 163: 287-303.
- García-Gómez C, Gortáres-Moroyoqui Py, Drogui P (2011) Contaminantes emergentes: efectos y tratamientos de remoció Química Viva 96-105.
- García-Gómez C, Drogui P, Zaviska F, Seyhi B, Gortáres-Moroyoqui P, et al. (2014) Experimental design methodology applied to electrochemical oxidation of carbamazepine using Ti/PbO2 and Ti/BDD electrodes. Journal of Electroanalytical Chemistry 732: 1-10.
- Rezaeifar Z, Es’haghi Z, Rounaghi GH, Chamsaz M (2016) Hyperbranched polyglycerol/graphene oxide nanocomposite reinforced hollow fiber solid/liquid phase microextraction for measurement of ibuprofen and naproxen in hair and wastewater samples. Journal of Chromatography B 1029-1030: 81-87.
- Zodi S, Potier O, Lapicque F, Leclerc J (2010) Treatment of the industrial wastewaters by electrocoagulation: Optimization of coupled electrochemical and sedimentation processes, Desalination. 261(1): 186-190.
- Farhadi S, Aminzadeh B, Torabian A, Khatibikamal V, Fard MA, et al. (2012) Comparison of COD removal from pharmaceutical wastewater by electrocoagulation, photoelectrocoagulation, peroxi-electrocoagulation and peroxi-photoelectrocoagulation processes. Journal of Hazardous Materials 219-220: 35-42.
- Zaroual Z, Chaair H, Essadki AH, El Ass K, Azzi M, et al. (2009) Optimizing the removal of trivalent chromium by electrocoagulation using experimental design. Chemical Engineering Journal 148(2-3): 488-495.
- Aleboyeh N, Daneshvar MB, Kasiri (2007) Optimization of C.I. Acid Red 14 azo dye removal by electrocoagulation batch process with response surface methodology. Chemical Engineering and Processing, 47(5): 827-832.
- Ahmadi M, Ghanbari F (2016) Optimizing COD removal from greywater by photoelectro-persulfate process using Box-Behnken design: assessment of effluent quality and electrical energy consumption. Environ Sci Pollut Res.

 Mini Review
Mini Review
