Abstract
Surface functionalization of inorganic nanoparticles without metal catalysts plays a vital role in their application, ranging from biomedical to biotechnological fields. Herein, we described a simple, two-step surface functionalization strategy for gold nanoparticles (AuNPs) using phase transfer and a metal-free ‘Thiol-Ene Click’ chemistry. Briefly, a tyrosine-capped photoproduced AuNPS were synthesized and transferred into a polar solvent using Octadecylamine (ODA) and further capped with Hexadecanedithiol (HDT). Then, the clickable layer and two different functional allyl derivatives of an acid (acrylic acid) and amine (Allylamine) were used to impart chemical functionality and different charge to the surface of AuNPs. Finally, physicochemical and surface properties of both pristine AuNPs and functionalized AuNPs have been examined by spectroscopic and microscopic techniques. The ease of synthesis for the thiol labeled AuNPs and its ability to undergo a very efficient “metal-free” bio-orthogonal, chemoselective thiolene “click” reaction would make a desirable platform for the covalent immobilization of biomolecules and biosensing probes.
Keywords: Gold Nanoparticles; Thiol-Ene Click; Metal-Free Bio-Orthogonal Click Reaction; Phase Transfer; Bioconjugation
Abbreviations: ODA: Octadecylamine; HDT: Hexadecanedithiol; DMF: Dimethylformamide; FTIR: Fourier Transforms Infrared
Introduction
Surface functionalized nanomaterials have emerged as one of the most critical research areas in the field of advanced functional materials [1,2]. These functional nanomaterials are designed to perform a specific role of scientific interest because of their interesting characteristics and widespread applications in drug delivery, medical diagnostics, catalysis, and many others applications [2-5]. By modifying the surface functional group or surface charge, one can modulate the dispersion and adsorption properties of nanoparticles and facilitate the immobilization of biological or non-biological moieties, including dyes, proteins, nucleic acids, fluorophores, Raman reporters, etc. [6]. For instance, Mirkin et al. used oligomers modified AuNPs (Spherical nucleic acid) for hybridization assay [7]. Murphy et al. employed functionalized AuNPs for colorimetric assay to monitor the detection of metal ions [8]. By incorporating the miRNA-specific oligonucleotide on the surface of AuNPs, Degliangeli et al. have designed gold nanoprobes to detect and quantify miRNAs [9]. On the other hand, Carmicheal and co-workers created an anti-MUC4 antibody, and Raman reporter conjugated AuNPs adduct to detect MUC4, a pancreatic cancer biomarker in serum sample [10]. Apart from these, the AuNPs with different functionality such as folate, peptide, and saccharides have been used for targeting and sensing applications.
All these applications emphasize the importance of surface functionality. However, chemistries involved in making these functional derivatives are also an area of tremendous interest that should include a straightforward, robust, and environmentally friendly synthetic route, which minimizes or eliminates the generation of any side products. Among many approaches, copper-catalyzed click (1,3-dipolar reaction) between azide and alkyne (Cu-AAC) has been used as an ideal route for the ligation of biomolecules. Research to date suggested that the Cu-AAC reaction has emerged as a valuable tool in biomedical fields and inorganic chemistry [11]. Click chemistry has resulted in a paradigm shift by displaying the practical implications of cycloaddition reactions on nanoparticle and cell surfaces, in the cell cytosol, or within the body, which is not easy with most other chemical reactions. Additionally, the Click chemistry in vitro approach allocated the specific labeling of cellular target proteins and investigated drug target engagement with drug surrogates in live cells. However, cytotoxicity of the Copper (Cu) catalyst has shown significant restriction on in vitro and in vivo applications [11-13]. To reduce this cytotoxic effect of copper, researchers have tried a variety of approaches.
It includes the use of stabilizing agents (i.e., bis(tertbutyltriazoly) ligand) or use of ring-strain (strain promoted azide-alkyne cycloaddition, SPAAC), which enables the increased reactivity of alkynes in an azide-alkyne reaction without the need for a cytotoxic copper. However, some chemists were unsatisfied with the second-order rate constant of the SPAAC reaction and the limitation of using large-sized Azide-alkyne molecules that can restrict the progress of the reaction. Thus, a simple alternative to strain-promoted cycloaddition reaction for the functionalization of nanoparticle or biomaterials without the use of copper catalyst is the need of the hour [14]. In the present study, we developed a simple, two-step surface functionalization strategy for gold nanoparticles (AuNPs) using phase transfer and a metal-free ‘Thiol-Ene Click’ (TEC) chemistry. We then demonstrated the ease of our TEC approach by clicking the Hexadecanedithiol (HDT) functionalized AuNPs (HDT@AuNPs) surface with allyl derivatives of an acid (acrylic acid) and amine (Allylamine). As a result, our approach imparted chemical functionality and charged on the surface of AuNPs via covalent conjugation. The generality of our assay opens up a new possibility for conjugations of different biological and biochemical analytes for biosensing, diagnostics, catalytic applications. Overall, we find this approach is straightforward, sitespecific, environmentally benign, and applicable to a wide range of analytes and biomolecules.
Materials and Methods
All chemicals and reagents were purchased from Sigma Alrich and used without any further purification unless otherwise mentioned.
Synthesis of Tyrosine-Capped AuNPs
Photochemical synthesis of Au NPs was carried out using a previously reported procedure with some modifications [15,16]. Briefly, a laboratory reactor system (UV consulting Peschel) fitted with a UV lamp (150 W medium pressure Hg lamp, Heraeus TQ 150) having quartz tubing for cooling with water. Under the vigorous stirring condition, the precooled KOH solution (10-3) was irradiated with a UV lamp. When the lamp glowed steadily at peak intensity, tyrosine and the HAuCl4 salt were added. Irradiation was continued for 20 min. The molar ratio of HAuCl4, tyrosine, and KOH was kept at 1: 3: 10.
Phase Transfer of Tyrosine-Capped AuNPs
Gold nanoparticles prepared by photoreduction method were phase transferred into an organic medium (i.e., chloroform) by using Octadecylamine (ODA) as a phase transfer agent. To a 50 mL as prepared Au NPs, 50 mL of 2x10-4 M ODA in chloroform was added, followed by 200 μL of 1 M HCl, and the mixture was agitated vigorously for a minute. The red wine color was observed in the lower organic phase while the upper aqueous phase becomes colorless. We recovered phase transferred AuNPs into an organic medium.
Thiol Functionalized Au NPs
Phase transferred Au NPs were further surface-functionalized using Hexamethylene Dithiol (HDT). In typical reaction to a 100 mL of AuNPs in CHCl3 (conc. Ca. ~10 nM) was sonicated for 15 min. The dispersion of Au NPs was mixed with a solution of HDT in CHCl3 (5 x 10-5 M). The reaction mixture was stirred vigorously at RT for 24 h under an inert atmosphere. At the end of the reaction, purification of AuNPs was carried using 3 rounds of centrifugation (15000 rpm, 30 min) interspersed and washing with ethanol. Finally, all AuNPs were dispersed in Dimethylformamide (DMF) and sonicated further for 30 min.
Thiol Click Reaction
In typical experiments, 50 mL of thiol functionalized AuNPs (thiol@Au NPs) dispersed in DMF (Conc. ca. ~ 5 nm) was taken in a 100 mL round bottom flask and sonicated for 15 min. Following complete dispersion of gold NPs, allylamine (Conc. Ca. 5 x 10-5 M) in DMF (1mL) was added dropwise under vigorous stirring and in an inert atmosphere for 24 h. At the end of the reaction, washing these NPs was carried out using two rounds of centrifugation (13000 rpm, 30 min) and three rounds of water (13000 rpm, 30 min) and redispersed in 10 mL of Mili-Q water. Following a similar procedure, thiol functionalized Au NPs also clicked with acrylic acid (5 x 10-5 M). Finally, all the above AuNPs were further characterized with UV- Vis spectroscopy on a carry 300 UV- Visible spectrometer at a resolution of 1 nm using a 5 mm path length quartz cuvette. Transmission Electron Microscopy (TEM) measurements were recorded by a JEOL Jem 1011 microscope (Accelerating voltage = 100kV). TEM analysis samples were prepared on carbon-coated copper grids (carbon 300 mesh Cu grid) by drop-casting Au NPs samples and adequately dried in a desiccator. Fourier transforms infrared (FTIR) spectra of Au samples were recorded on Bruker Vertex-70 FT-IR spectrometer. All FTIR samples were prepared by making a KBr pellet of Au NPs and dried well under a vacuum desiccator overnight.
Results and Discussion
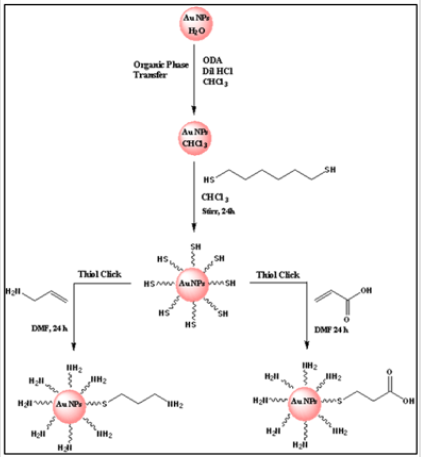
Gold nanoparticles (AuNPs) are valuable backbone materials for developing sensing systems and bioimaging techniques in biology, biotechnology, and nanomedicine [17-22]. During the fabrication process, an important role is played by the functionalization of the NP surface, frequently achieved with thiol ligands. For instance, this step is used to impart water solubility to the particles [23- 28], an essential feature for applying AuNPs in physiologically relevant media. Functionalized nanoparticles that specifically interact with cellular targets are of great interest in biotechnology, biomedicine, and biodiagnostic development [22,29,30]. Especially, functionalized gold nanoparticles (AuNPs) have been demonstrated as versatile tools for various biotechnological applications [17- 19,31]. For instance, cell-penetrating peptides conjugated with AuNPs to improve internalization, [32-35] peptides to target the cell nucleus, [34,36-38] oligonucleotides–functionalized AuNPs used in the development of a biosensor for DNA analysis, [39] cell targeting, [40] aptamer conjugated–AuNPs in making biosensor strip for protein analysis, [41] indicating the selectivity and versatility of the biomolecule functionalized nanoparticles. Understanding the importance of surface functionalization, we have developed a new synthetic route to modify AuNPs surface with both acid and amine functional group using the Thiol-ene Michael addition method (Figure 1) and characterized them with spectroscopic and microscopic techniques.
Figure 1: Representative scheme for functionalization of 5 nm AuNPs using Thiol-ene click (Michael) addition reaction.
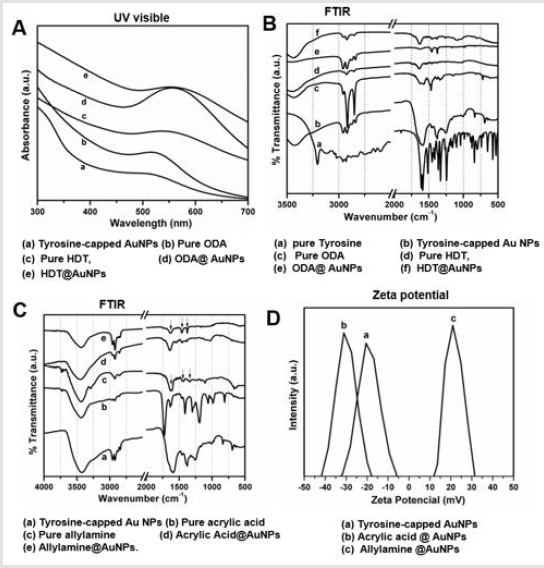
To study the stability and surface plasmon resonance of the AuNPs during the course reaction, we collected UV-visible spectra of AuNPs. In Figure 2A, SPR spectra of photoreduction AuNPs were observed at 510 nm (curve a), indicating the characteristic plasmonic resonance band for Au NPs solution. After phase transfer of Au NPs with ODA in CHCl3, a distinct redshift at wavelength 525 nm (curve b) can be observed. Further treatment of ODA@Au NPs with HDT in CHCl3 shows absorbance at 542 nm (curve C). One thing to be considered here that while comparing the UV- Vis spectra of ODA and HDT-capped AuNPs with respect to as-prepared Au NPs show a distinct red shift in absorbance peaks. This shift is well known and is attributed to the changes in the electron cloud at the Au NP surface due to chemisorption of amine or thiol functionalized capping agents. Further to understand surface functionality, we performed FTIR measurement of all ODA and HDT-capped (Figure 2B) and Acid/amine-functionalized AuNPs (Figure 2C). Evidently, the characteristic peak of -C=O stretch at 1680 cm-1 from acrylic acid is retained acid-functionalized AuNPs. However, the peaks observed between 3000-2850 cm-1 attributed to the -C-H stretch and large featured centered near 1670 cm-1 for allylamine-capped AuNPs are consistent with -N-H stretch of pure allylamine addition peak observed between 1500 and 1400 cm-1 stretched for C-C bending modes. Overall, the FTIR results revealed surface functionalization of acid and amine moieties. To further confirm these results, we determined the surface charge of AuNPs (Figure 2D). Interestingly, both the tyrosine and acrylic acid capped AuNPs showed negative zeta potential -15 mV and -27 mV due to the exposed -COO¯ group at the outer surface; whereas, allylamine capping AuNPs displayed a drastic change in the surface charge of 22 mV, confirming the successful collating of the allylamine.
Figure 2:Representative spectroscopic analysis showing surface characterization of AuNPs
A. UV visible spectra of AuNPs and phase transferred AuNPs
B. FTIR spectra of AuNPs and phase transferred AuNPs
C. FTIR spectra of AuNPs and acid and amine functionalized AuNPs
D. Zeta potential measurement of acid and amine functionalized AuNPs.
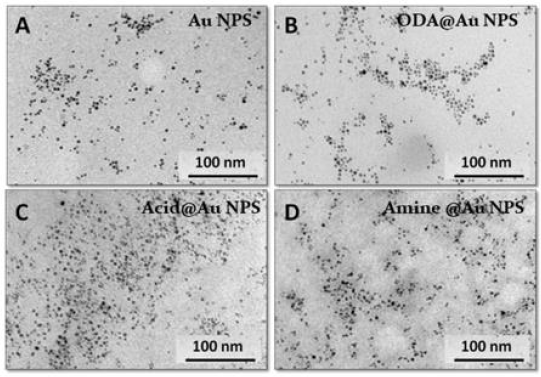
Next, to confirm the size, morphology, and stability (i.e., aggregation status) of AuNPs before and after the coating/ functionalization, the TEM imaging was carried out (Figure 3). At first glance, it can be observed that all the samples of before and after surface modification the NPs seem to have a narrow size distribution. From the Gaussian fit of the histogram, the mean particle size were calculated to be between 4.0-6.0 nm. In summary, our characterization data revealed a successful synthesis of acidand amine-functionalized AuNPs using the thiol-ene click chemistry. Collectively, the ease of synthesis for the thiol-labeled AuNPs and its ability to undergo a very efficient “metal-free” bio-orthogonal, chemoselective thiol-ene “click” reaction would make an attractive platform for its covalent immobilization of biomolecules and biosensing probes.
Figure 3:TEM images of
A. Tyrosine-capped AuNPs
B. ODA@AuNPs
C. Acid-functionalized AuNPs
D. Amine-functionalized AuNPs (Scale bar 100 nm).
Conclusion
In summary, we have shown the application of the Thiolene click chemistry approach as an efficient way to impart both acid and amine functionality on the surface of AuNPs. We have demonstrated that the thiol-coated and phase transferred AuNPs are participating in the bio-orthogonal Thiol-ene click reaction and imposing the functionality of the acid-/amine group in the presence of a polar solvent. Further, we checked the stability, surface charge, and functionality of the AuNPs using UV visible, zeta potential, and FTIR spectroscopy. The TEM imaging of functionalized AuNPs revealed a formation of stable, monodispersed AuNPs with narrow size distribution and without any significant traces of aggregation. The simplicity of the Thio-ene modified AuNPs opened up a new future perspective in designing a more rational strategy for conjugating various analytes and biomolecules to metal nanoparticles and surfaces for biosensing, biomarker diagnostics, and other bioimaging applications.
Conflicts of Interest
All other authors declare no competing financial interest.
References
- Song JH, Min SH, Kim SG, Cho Y, Ahn SH (2021) Multi-functionalization Strategies Using Nanomaterials: A Review and Case Study in Sensing Applications. International Journal of Precision Engineering and Manufacturing-Green Technology.
- Wieszczycka K, Staszak K, Woźniak-Budych MJ, Litowczenko J, Maciejewska BM, et al. (2021) Surface functionalization – The way for advanced applications of smart materials. Coordination Chemistry Reviews 436(1): 213846.
- Chen W, Zhang J, Wei X (2021) Functional Nanomaterials for Cancer Diagnostics and Therapy. Front Chem 9: 670410.
- Franco R, Pedrosa P, Carlos FF, Veigas B, Baptista PV (2015) Gold Nanoparticles for DNA/RNA-Based Diagnostics. Handbook of Nanoparticles, pp. 1339-1364.
- Perinot A, Kshirsagar P, Malvindi MA, Pompa PP, Fiammengo R, et al. (2016) Direct-written polymer field-effect transistors operating at 20 MHz. Scientific Reports 6(1): 38941.
- Shen J, Li Y, Gu H, Xia F, Zuo X (2014) Recent development of sandwich assay based on the nanobiotechnologies for proteins, nucleic acids, small molecules, and ions. Chem Rev 114(15): 7631-7677.
- Cutler JI, Auyeung E, Mirkin CA (2012) Spherical Nucleic Acids. Journal of the American Chemical Society 134(3): 1376-1391.
- Obare SO, Hollowell RE, Murphy CJ (2002) Sensing Strategy for Lithium Ion Based on Gold Nanoparticles. Langmuir 18(26): 10407-10410.
- Degliangeli F, Kshirsagar P, Brunetti V, Pompa PP, Fiammengo R (2014) Absolute and Direct MicroRNA Quantification Using DNA–Gold Nanoparticle Probes. Journal of the American Chemical Society 136(6): 2264-2267.
- Carmicheal J, Hayashi C, Huang X, Liu L, Lu Y, et al. (2019) Label-free characterization of exosome via surface enhanced Raman spectroscopy for the early detection of pancreatic cancer. Nanomedicine: nanotechnology, biology, and medicine 16: 88-96.
- Meldal M, Tornøe CW (2008) Cu-Catalyzed Azide−Alkyne Cycloaddition. Chemical Reviews 108(8): 2952-3015.
- Amblard F, Cho JH, Schinazi RF (2009) Cu(I)-Catalyzed Huisgen Azide−Alkyne 1,3-Dipolar Cycloaddition Reaction in Nucleoside, Nucleotide, and Oligonucleotide Chemistry. Chemical Reviews 109(9): 4207-4220.
- Suazo KF, Park KY, Distefano MD (2021) A Not-So-Ancient Grease History: Click Chemistry and Protein Lipid Modifications. Chemical Reviews 121(12): 7178-7248.
- Kim E, Koo H (2019) Biomedical applications of copper-free click chemistry: in vitro, in vivo, and ex vivo. Chemical Science 10(34): 7835-7851.
- Kshirsagar P, Sangaru SS, Brunetti V, Malvindi MA, Pompa PP (2014) Synthesis of fluorescent metal nanoparticles in aqueous solution by photochemical reduction. Nanotechnology 25(4): 045601.
- Kshirsagar P, Sangaru SS, Malvindi MA, Martiradonna L, Cingolani R, et al. (2011) Synthesis of highly stable silver nanoparticles by photoreduction and their size fractionation by phase transfer method. Colloids and Surfaces A: Physicochemical and Engineering Aspects 392(1): 264-270.
- Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, et al. (2008) Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc Chem Res 41(12): 1721-1730.
- Baptista P, Pereira E, Eaton P, Doria G, Miranda A, et al. (2008) Gold nanoparticles for the development of clinical diagnosis methods. Anal Bioanal Chem 391(3): 943-950.
- Sperling RA, Gil PR, Zhang F, Zanella M, Parak WJ (2008) Biological applications of gold nanoparticles. Chem Soc Rev 37: 1896-1908.
- Wilson R (2008) The use of gold nanoparticles in diagnostics and detection. Chem Soc Rev 37: 2028-2045.
- Jain PK, El-Sayed IH, El-Sayed MA (2007) Au nanoparticles target cancer. Nano Today 2(1): 18-29.
- Alivisatos P (2004) The use of nanocrystals in biological detection. Nat Biotechnol 22(1): 47-52.
- Uzun O, Hu Y, Verma A, Chen S, Centrone A, et al. (2008) Water-soluble amphiphilic gold nanoparticles with structured ligand shells. Chem Commun, pp. 196-198.
- Gentilini C, Evangelista F, Rudolf P, Franchi P, Lucarini M, et al. (2008) Water-Soluble Gold Nanoparticles Protected by Fluorinated Amphiphilic Thiolates. J Am Chem Soc 130(46): 15678-15682.
- Pengo P, Baltzer L, Pasquato L, Scrimin P (2007) Substrate modulation of the activity of an artificial nanoesterase made of peptide-functionalized gold nanoparticles. Angew Chem Int Ed 46(3): 400-404.
- Foos EE, Snow AW, Twigg ME, Ancona MG (2002) Thiol-Terminated Di-, Tri-, and Tetraethylene Oxide Functionalized Gold Nanoparticles: A Water-Soluble, Charge-Neutral Cluster. Chem Mater 14(5): 2401-2408.
- Kanaras AG, Kamounah FS, Schaumburg K, Kiely CJ, Brust M (2002) Thioalkylated tetraethylene glycol: a new ligand for water soluble monolayer protected gold clusters. Chem Commun, pp. 2294-2295.
- Shon YS, Wuelfing WP, Murray RW (2001) Water-Soluble, Sulfonic Acid-Functionalized, Monolayer-Protected Nanoparticles and an Ionically Conductive Molten Salt Containing Them. Langmuir 17(4): 1255-1261.
- De M, Ghosh PS, Rotello VM (2008) Applications of Nanoparticles in Biology. Adv Mater 20(22): 4225-4241.
- Rosi NL, Mirkin CA (2005) Nanostructures in biodiagnostics. Chem Rev 105(4): 1547-1562.
- Boisselier E, Astruc D (2009) Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev 38: 1759-1782.
- Pujals S, Bastus NG, Pereiro E, Lopez-Iglesias C, Puntes VF, et al. (2009) Shuttling gold nanoparticles into tumoral cells with an amphipathic proline-rich peptide. ChemBioChem 10(6): 1025-1031.
- Ryan JA, Overton KW, Speight ME, Oldenburg CN, Loo L, et al. (2007) Cellular uptake of gold nanoparticles passivated with BSA-SV40 large T antigen conjugates. Anal Chem 79(23): 9150-9159.
- Liu Y, Shipton MK, Ryan J, Kaufman ED, Franzen S, et al. (2007) Synthesis, Stability, and Cellular Internalization of Gold Nanoparticles Containing Mixed Peptide−Poly(ethylene glycol) Monolayers. Anal Chem 79(6): 2221-2229.
- Sun L, Liu D, Wang Z (2008) Functional Gold Nanoparticle−Peptide Complexes as Cell-Targeting Agents. Langmuir 24(18): 10293-10297.
- Patel PC, Giljohann DA, Seferos DS, Mirkin CA (2008) Peptide antisense nanoparticles. Proc Natl Acad Sci USA 105(45): 17222-17226.
- Oyelere AK, Chen PC, Huang X, El-Sayed IH, El-Sayed MA (2007) Peptide-Conjugated Gold Nanorods for Nuclear Targeting. Bioconjugate Chem 18(5): 1490-1497.
- Tkachenko AG, Xie H, Liu Y, Coleman D, Ryan J, et al. (2004) Cellular trajectories of peptide-modified gold particle complexes: comparison of nuclear localization signals and peptide transduction domains. Bioconjug Chem 15(3): 482-490.
- Glynou K, Ioannou PC, Christopoulos TK, Syriopoulou V (2003) Oligonucleotide-Functionalized Gold Nanoparticles as Probes in a Dry-Reagent Strip Biosensor for DNA Analysis by Hybridization. Analytical Chemistry 75(16): 4155-4160.
- Maus L, Dick O, Bading H, Spatz JP, Fiammengo R (2010) Conjugation of Peptides to the Passivation Shell of Gold Nanoparticles for Targeting of Cell-Surface Receptors. ACS Nano 4(11): 6617-6628.
- Xu H, Mao X, Zeng Q, Wang S, Kawde AN, et al. (2008) Aptamer-Functionalized Gold Nanoparticles as Probes in a Dry-Reagent Strip Biosensor for Protein Analysis. Analytical Chemistry 81(2): 669-675.

 Short Communication
Short Communication