ABSTRACT
Trichinellosis is a zoonosis of worldwide distribution that affects humans, and is transmitted by mammals, birds and reptiles. The World Health Organization ranked it in the seventh place of food-borne diseases in 2014. In Zacatecas Mexico it has been an endemic zoonosis since 1978. There are different drug treatments such as mebendazole, albendazole, and prolonged treatments with side effects at the liver and other organs. Rifampicin is a broad-spectrum antibiotic, it inhibits the growth of numerous mycobacteria, both typical and atypical, Gram-positive and Gram-negative bacteria. Objective: In this study was evaluated the usefulness of rifampicin in Trichinella spiralis infection in a murine experimental model in the intestinal phase. Results: By the A /D and HE techniques, modification of the morphology of the Infectious larvae (IL) of Trichinella spiralis, and the statistical study regarding the parasite load, a P-value of 0.001 was found, showing that this factor is statistically significant on the effect of feasibility with a confidence level of 95%. Conclusion: Rifampicin had an effect on the infecting larvae of Trichinella spiralis in the intestinal phase.
Abbreviations: IL: Infectious Larvae; NLR: Newborn Larvae; SAGARPA: Secretariat of Agriculture, Livestock , Rural Development, Fisheries and Food; pn: Polymorphonuclear Cells
Introduction
Trichinellosis is a parasitic disease and an endemic zoonosis in various countries of the world, it is caused by the genus Trichinella and its 12 genotypes, due to the consumption of contaminated and poorly sewn meat with Infectious Larvae (IL), the diagnosis is not made in a usual way and is confused with other pathologies [1], in humans the transmission is primarily by pig [2], and it is found in other domestic animals such as dogs [3], horses, domestic rats [4]. However, in the last 10 years the incidence of infection due to consumption of wild boar, fox, walrus and bear, and puma have been present [5]. Zacatecas Mexico has been considered an endemic area of the disease since 1978, when the first outbreak occurred in the municipality of Villanueva [6], and further outbreaks have been reported since then until present date [7]. In several countries of the world including Spain, Argentina, Chile and Mexico, the transmitting species is Trichinella spiralis, its life cycle is made up of 3 stages: First, human consume contaminated pork with infectious larvae of T. spiralis, then 30 hours later male and female larvae mature into adult stage being localized in the small intestine producing the fertilization process, later on the fifth day the birth of Newborn Larvae (NLR) happen [8], finally they pass into circulation through the portal system and from there to the striated muscle where they implant, and form the nurse cell [9].
The diagnosis of the disease is made by direct plate compression techniques (P/C), Artificial Digestion (A/D), Hematoxylin-Eosin (H/E), Polymerase Chain Reaction (PCR), and indirect techniques such as DOT-ELISA , ELISA and Wester-Blot [10,11]. One of the first drugs used was mebendazole, currently its use is little [12]. Various antiparasitics such as albendazole, nitazoxanide, ivermectin, quinfamide, have been evaluated in experimental models, being effective [13,14], with the disadvantage that they have long administration times causing adverse reactions in the host [15]. Likewise, there are alternative drugs such as resiniferatoxin which have an anti-inflammatory effect at the intestinal level [16-17], and Tamoxifen which decrease the parasitic load of T. spiralis [18]. Another alternative treatment is the use of immunogens, total extracts of the parasite, the 45 kDa antigen, and different routes of administration have been evaluated, using the 45 kDa antigen being effective sublingually [19-22]. Rifampicin belongs to a group of structurally similar complex macrocyclic antibiotics produced by Streptomyces mediterranei [23]. This drug is a broad-spectrum bactericidal antibiotic used in the treatment of infections caused by mycobacteria, mainly M. tuberculae and M. leprae, and is also indicated in the treatment of Neisseria meningitidis infections. Its main interest lies in its bactericidal power and its excellent tissue and intracellular diffusion. Rifampicin is a powerful enzyme inducer: its main drawback is the risk of drug interactions, it is generally marketed in the form of 150 and 300 mg capsules with a maximum dose of 600 mg, although it can also be presented in other pharmaceutical forms, such as, rifampicin 1% oral suspension and 300 mg suppositories [24-26]. It should be restricted in patients with liver failure, and pregnant women as it crosses the placenta.
Objective
To evaluate the usefulness of Rifampicin in T. spiralis infection in a murine experimental model, in the intestinal phase.
Justification
Trichinellosis is an endemic zoonosis reported as a reemerging disease, an increased number of cases have been observed in humans and animals, in Mexico some studies show the presence of the disease in practically the 32 states of the Mexican Republic, unfortunately it is not done the adequate diagnose, due to the little information on the clinical background. Some treatments have had adequate results, such as albendazole in the intestinal and muscular phase, but it has the disadvantage of producing collateral effects: Hepatotoxic, Teratogenic and it is a 14-day treatment. Some antigens have been tested with good results, but unfortunately, they have not crystallized as vaccines. Hence the interest of having a treatment with fewer side effects and shorter dose administration time. Rifampicin is bactericidal against intracellular and extracellular forms, it penetrates phagocytic cells, in the intestine it enters the enterohepatic circulation, and its toxicity is low. Based on these properties it may have an effect on T. spiralis.
Materials and Methods
Rats used in the experiments were obtained from the animal facility of the Academic Unit of Biological Sciences of the Autonomous University of Zacatecas. The parasite (Mexican strain) was identified with Edoardo Pozio PhD, in the Istituto Superiore di Sanita in Rome, Italy 2000, and has been maintained by serial passage in mice and rats since 1979 at the Laboratory of Cell Biology and Microbiology at the Academic Unit of Biological Sciences from the Autonomous University of Zacatecas. Zacatecas, Mexico. All the animals were maintained in controlled-temperature rooms and fed with rodent balanced food [2,16,17]. Parameters evaluated: parasite load using the direct artificial digestion technique (A/D), characteristics of the nurse cell using the plate compression technique (P/C) and Hematoxylin Eosin staining (H/E) and indirect techniques the Immune response by Western Bloot, statistical analysis was performed with the Strargraphics 5.1 program. Ethical approva:l This study was reviewed and approved by the Bioethics Committee of the Biology Faculty of the Autonomous University of Zacatecas, in accordance with the Official Mexican Norm (NOM-062- ZOO-1999), published by the Secretariat of Agriculture, Livestock , Rural Development, Fisheries and Food (SAGARPA) in the Official Gazette of the Federation (Mexico) on June 28, 2001. [27,28].
Experimental Murine Model
The experimental murine model used was Long Evans rats of two and a half months of age, divided into 5 groups of 10 animals each, all were infected orally with 500 Infecting Larvae (IL) of T. spiralis. Group one: control of infection without treatment, sacrificed after 30 days. Group two: start of treatment on day one of infection. Group three: start of treatment after 7 days of infection. Group four: start of treatment on day 15 of infection. Group five: start of treatment at 30 days infection, Rifampicin Treatment (TX) with adjusted human dose for rats, 6.0 Units (U.) (5 mg/kg of weight) for ten days and sacrificed at 20 after the TX was finished (Table 1). Parameters evaluated: body weight and height (at the beginning of the experiment and at the sacrifice of the animals of all study groups), blood sampling from the retro-orbital sinus in the pre-infection with T. spiralis and at sacrifice, the Parasitic load using the direct plate compression technique (P/C), artificial digestion (A/D), and Hematoxylin Eosin (H/E) staining and the indirect immunological response technique by Western Blot (WB).
Plate Compression Technique
For the plate compression technique, approximately 5 mg of tissue were used (diaphragm, masseter, tongue, intercostals, leg), each sample was placed between 2 lamellae and compressed, occupying an area of 1 x 5 mm, it was observed to the optical microscope, with the 10x and 40x lens [1,3,4].
Artificial Digestion Technique
30 g samples were used of homogenized tissue, and they were incubated at 37 °C, in a sack-shaped tulle sieve, suspended in a 0.3% solution of pepsin (10,000 U) and 37% HCl (0.2M) in 500 ml of distilled water , inside a separating funnel; 24 hours later, the larval package was separated with the ILs, which were deposited at the bottom of the funnel, observed in a new bawer camera under an optical microscope with a 10X lens, and the larval package was quantified [1,3,4].
Hematoxylin-Eosin staining
The tissue was preserved in 10% formalin for histological sections, in order to determine the characteristics by a Hematoxylin- Eosin (H/E) stain. The tissues were dehydrated to process them in paraffin, this was carried out in a tissue processor (Lipshaw Automatic Tissue Processor) as follows: The tissue remained in 10% formaldehyde for 12 hours and in 80% ethyl alcohol for 1 hour, then 96% ethyl alcohol, making 3 changes of 30 min. and absolute ethyl alcohol, 3 changes every hour and a half, then Xylene, 2 changes every 1.5 hours and, finally, paraffin at 60 °C, in 2 changes every hour and a half and at the end the tissues were placed in cubic molds until cool. After 24 hours, they were placed on an ice bed to make the cuts, with a thickness of 4m (Microtome model 820 Rotary, American Óptica), later they were placed on a slide, then covered with a gel based on egg white and glycerol in a 1: 1 dilution, plus some grains of Tibol as a preservative, the sections were placed on a hot plate to melt the paraffin and continue with the staining with H/E. The staining process was carried out in a staining train: the slides were placed in a basket that was introduced into the following solutions: Xylene, 5 min; absolute ethyl alcohol, 3 min; 96% ethyl alcohol, 3 min; 96% ethyl alcohol 3 min; distilled water (quickly); Harris Hematoxylin (for a few seconds) drinking water, 1% acid alcohol (fast) drinking water; saturated lithium carbonate solution, 2min .; drinking water-Eosin (for a few seconds); drinking water, two changes in 96% ethyl alcohol; 3 changes in absolute ethyl alcohol, Xylene (all previous steps are 1 min) Were mounted with Sigma â synthetic resin and covered with a slide, ready for observation under the light microscope at 40 and 100X [29].
Western Blot (WB)
The product obtained from the polyacrylamide gel run was transferred to NC paper [30], using the Transblot-Cell camera (Bio- Rad) at 35 volts, overnight at 4 °C. The NC paper was dyed with fast green for 5 min. With constant stirring, the dye was removed and decolorized in distilled water, to verify protein transfer, it was allowed to dry and the strips of the approximate width of each lane (0.5 cm) were cut. After the above, each strip was covered with a solution of PBS-3% milk powder and 0.15% sodium azide at 4 °C, with constant stirring overnight. They were then washed 3 times for 10 min. with PBS, incubation was continued for 1.5 h. with the sera of the rats in a dilution of 1: 100 in PBS-3% milk powder at 37 °C with constant agitation, subsequently they were washed twice with PBS-0.3% Tween 20 for 10 min and three more with PBS for another 10 min. Then, they were incubated with the second antirat IgG antibody, conjugated with peroxidase 1: 2000 PBS-3% milk powder for 1 h., At room temperature, with shaking, then they were washed 2 times with PBS-Tween 20 at 3% and rinsed with PBS, for 10 min. The Banding pattern of each strip was developed with 3,3´ di amino-benzidine (DAB), 50 mg in 100 mL of PBS, using, as a substrate, 37% hydrogen peroxide [31]. The present study was analyzed with the Strargraphics 5.1 program in which the result of the analysis of variance was obtained.
Results
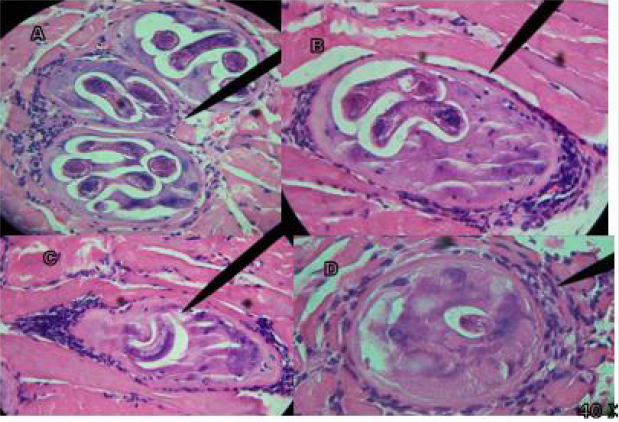
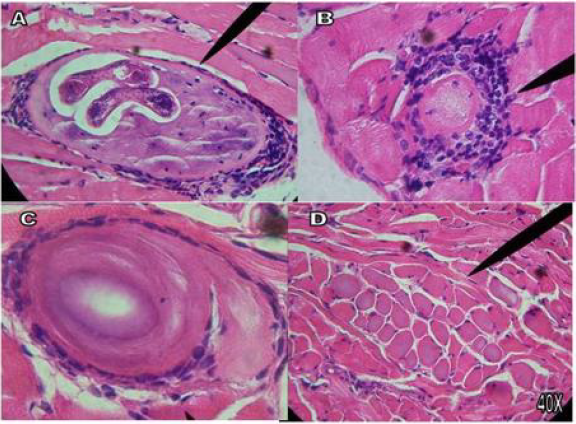
In the plaque compression, in the control group the welldefined nurse cell is observed and within it the IL with its own spiral characteristics, in the treated groups the absence and presence of the nurse cell with alterations and morphological modification of the IL. In the D/A, in the control group the ILs with their spiral morphology, their esticosome and their well-defined posterior and anterior part are observed, in the four treated groups fragments of the parasite are observed, exit from their interior content, loss of its characteristic spiral morphology, residues and fragments of T. spiralis. In the H/E staining, in the infected control group T. spiralis is observed inside the nurse cell, as well as the presence of polymorphonuclear cells (pn), in the four treated groups the absence and presence of modified IL is observed, regeneration tissue, modified nurse cell, IL fragments, and presence of nuclear polymorphs (Figures 1-4).
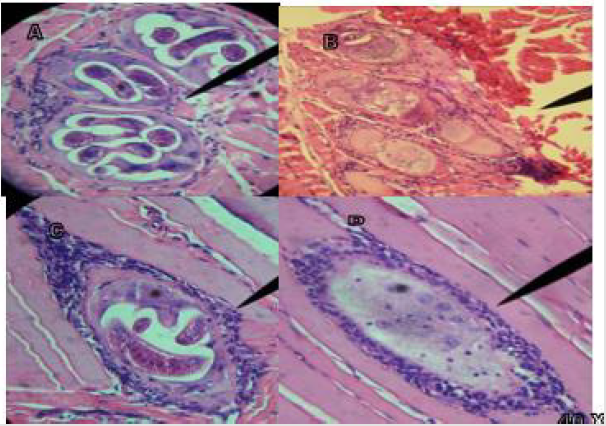
Figure 1A shows infected masseter tissue at 40 X of the control group, where the presence of polymorphonuclear (pn) T. spiralis ILs and their characteristic nurse cell are observed; Figure 1B shows infected tongue tissue at 40 X of the control group, where the presence of the IL of T. spiralis, its characteristic and polymorphonuclear (pn) nurse cell, is observed; In figure C and D at 40 X, infected leg tissue is shown, in Figure 1C the presence of the IL of T. spiralis, its characteristic nurse cell and polymorphonuclear cells (pn) is observed; Figure 1D shows the start of implantation of the IL. Tissues stained with Hematoxylin-Eosin stain. Figure 2A shows infected masseter tissue at 40 X of the control group, where the presence of the IL of T. spiralis, its characteristic nurse cell and polymorphonuclear cells (pn) is observed; Figure 2B shows infected masseter tissue at 40 X from group one which was treated with Rifampicin on the first day post-infection, where absence of IL, nurse cell and tissue regeneration are observed. Figures 2C & 2D show infected leg tissue from group one, which was treated with Rifampicin the first day after infection, Figure C shows the presence of the IL at 40 X, its nurse cell and polymorphonuclear cells (pn), in Figure D at 40X, the absence of IL and pn is observed. Tissues stained with Hematoxylin - Eosin stain. Figure 3A shows infected tongue tissue at 40 X of the control group, where the presence of the IL of T. spiralis, its characteristic nurse cell and polymorphonuclear cells (pn) is observed; Figure 3B shows infected masseter tissue at 40 X where a fragment of IL, the nurse cell and absence of pn are shown. Figure 3C shows infected tongue tissue at 40 X where absence of IL and tissue regeneration are shown; Figure 3D shows infected leg tissue at 40 X where the absence of IL is observed, modification in the nurse cell with tissue regeneration. Tissues stained with Hematoxylin-Eosin stain.
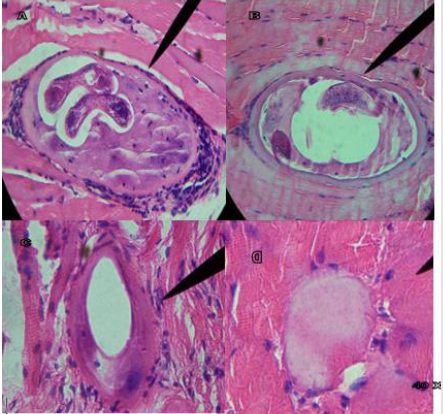
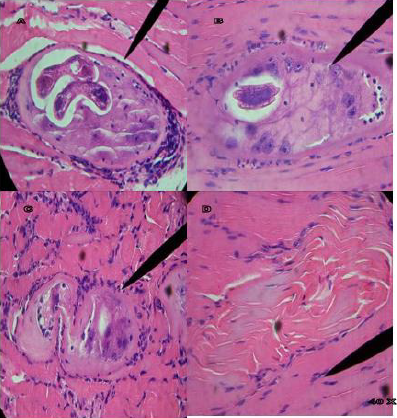
Figure 4A shows infected tongue tissue at 40 X of the control group, where the presence of the IL of T. spiralis, its characteristic nurse cell and polymorphonuclear cells (pn) is observed; Figure 4B at 40 X shows masseter tissue where a fragment of IL is observed inside the nurse cell and pn; Figure 4C at 40 X shows masseter tissue where the absence of IL is observed, and the modified nurse cell with tissue regeneration; Figure 4D shows tissue regeneration in the place where the presence of an IL was found; Tissues stained with Hematoxylin-Eosin stain. Figure 5A shows infected tongue tissue at 40 X of the control group, where the presence of the IL of T. spiralis, its characteristic nurse cell and polymorphonuclear cells (pn) is observed; Figure 5B shows infected tissue of the diaphragm at 40 X where recovery tissue is observed in the place where the presence of an IL and pn probably existed; Figures 5C & 5D show infected tongue tissue at 40X, Figure 5C shows the absence of IL, pn and tissue regeneration; Figure 5D shows tissue in recovery in the place where the IL was present. WB the characteristic triplet of T. spiralis parasitosis, 42, 45 and 48 kDa, is observed.
Results Obtained in the Statistical Analysis
The present study was carried out with the Strargraphics 5.1 program in which the result of the analysis of variance was obtained in order to determine a statistically significant result on Viability, the result of the P-value is 0.001 showing that this factor is statistically significant on the feasibility effect with a confidence level of 95%.
Discussion
Several authors [13,14] have evaluated different antiparasitics such as albendazole, ivermectin, nitaxozamide, quinfamide, having the best effect with albendazole, but applying a 14-day treatment and have presented collateral effects such as hepatotoxicity and teratogenicity [15]. Likewise, other drugs such as resiniferatoxin and tamoxifen have been evaluated [16-18]. Rifampicin, which is an antibiotic that acts at the intracellular level, was found to have a statistically significant efficacy in the parasitic load of T. spiralis and that the tissue recovers its physiological state. The treatments were established at day 1, 7.15 and 30 of infection, in all of them there were modifications of the nurse cell, on days 1.7 and 15 the parasite is in the intestinal phase [8], which is indicative that the life cycle of the parasite in its stage of IL, adults and LRN, and on day 30 the parasite is already in the muscular phase, but the 10 days of treatment, and the subsequent 20 until sacrifice had an effect on the IL already implanted. Opening a line of research to evaluate the mechanism of rifampicin damage on the parasite and standardize if it is effective with less doses.
Trichinellosis is a zoonosis with a worldwide distribution, which have increased the species, genotypes [32], hosts and susceptibles in acquiring this parasitic disease, in Europe Trichinella spiralis circulates in domestic and wild hosts such as pigs, domestic rats, wild boars, and in red fox Trichinella britovi [33], in Mexico the predominant one in pig is Trichinella spiralis, and so far it has not been reported in wild boars, however with the decrease in rural areas, its hunting and human consumption is more frequent. We currently have better diagnostic techniques, however, it is considered pertinent to improve their quality. Artificial digestion [34] is essential in health diagnoses that samples of a gram of meat are reliable for diagnosis. Taking these elements from there our interest to have reliable treatments and do not have collateral effects, and to continue working on the crystallization of a vaccine [19-23].
Conclusion
Despite the fact that Rifampicin inhibits the growth of numerous bacteria and is bactericidal against intracellular and extracellular forms, the results obtained in the present work show that rifampicin is effective in Tx. against T. spiralis in the intestinal phase, since it had a direct action on the IL, the result is supported by each of the results obtained in the different tests that were carried out and with the P-value result of 0.001 and 95% reliability.
References
- Moreno García MA (2018) Epidemiología, diagnóstico y tratamiento de la Trichinellosis en Mé 2018. Editorial Académica Española, p. 1-127.
- Chávez Ruvalcaba MI, Reveles Hernández RG, Muñoz Escobedo JJ, Maldonado Tapia C, Moreno García MA (2011) Utilidad del modelo experimental de cerdo en el estudio y tratamiento de la Trichinellosis. REDVET 12(5B): 1-18.
- Berumen de la Torre V, Muñoz Escobedo JJ, Moreno García MA (2002) Trichinellosis en perros callejeros de la ciudad de Zacatecas, Mé Parasitología latinoamericana. 57(1-2): 72-74.
- Moreno GA, Rivas GJ, Berumen TV, Muñoz EJ (2007) Detección de Trichinella spiralis en rata domestica del basurero municipal de Zacatecas. REDVET 8(5): 1-8.
- Waits A, Emelyanova A, Oksanen A, Abass K (2018) Human infectious diseases and the changing climate in the Arctic. Environ Int Internet 121: 703-713.
- Fragoso Uribe R (1981) Un brote de triquinosis en Villanueva, Zacatecas. Sal Públ Méx 23(1): 25-41.
- Maldonado Tapia C, Bracamontes Maldonado N, López Bernal S, Muñoz Escobedo J, Chávez Guajardo E, Moreno García (2015) A Anti-T spiralis Antibodies Detection in some Localities of Zacatecas (México). International Archives of Medicine 8(216):1-6.
- Moreno García MA, Maldonado Tapia CH, García Mayorga EA, Reveles Hernández RG, Muñoz Escobedo JJ (2009) Fase Intestinal de Trichinella spiralis en modelo murino. Acta Biológica Colombiana 14(1): 203-210.
- Muñoz Carrillo JL, Maldonado Tapia C, López Luna A, Muñoz Escobedo JJ, Flores De La Torre JA (2018) Current aspects in Trichinellosis. In: Bastidas Pacheco GA (Edt.)., Parasites and Parasitic Diseases. London, UK. IntechOpen, p. 65-87.
- Aguilar BR, Bautista G, Rojas J, De Nova ME, Ixta O, et al. (2000) Experimental swine trichinellosis: use of Dot_ELISA and Western Blot with excretion/secretion antigens (ES) from infective larvae to detect anti-Trichinella spiralis antibodies. Rev Latinoam Microbiol 42: 57-62.
- Moreno García MA, Muñoz Escobedo JJ, Reveles Hernández RG, Saldivar Elías S, Vació De La Torre MDR (2007) Utilización de técnicas directas e indirectas en el diagnóstico de Trichinellosis en cerdo. Sitio Argentino de Producción Animal, p. 1-9.
- Flórez Jesús (2000) Fármacos antiparasitarios II. Helmintos y artrópodos en Farmacología Humana, tercera edición, editorial masson SA, pp. 1239-1245.
- Chávez Ruvalcaba I, Reveles Hernández G, Saldivar Elías S, Muñoz Escobedo J, Morales Vallarta M, Moreno García A (2006) Evaluation of 3 antiparasites on intestinal and muscular phase infection of Trichinella spiralis in the pig model. REDVET 7(6): 1-8.
- García Robles MJ, Reveles Hernández G, Saldivar Elías S, Muñoz Escobedo JJ, Moreno García MA (2011) Utilidad del Alabendazol/Quinfamida en el tratamiento de la fase intestinal de la infección por Trichinella spiralis en modelo murino. AVFT 20(1): 1-5.
- Chávez Guajardo EG, Morales Vallarta MR, Saldivar Elías SJ, Reveles Hernández RG, Muñoz Escobedo JJ, et al. (2010) Detección de los cambios fenotípicos en productos de ratas Long Evans infectadas con Trichinella spiralis y tratadas con Albendazol. Archivos Venezolanos de Farmacología Terapéutica 29(2): 29-31.
- Muñoz Carrillo JL, Muñoz Escobedo JJ, Maldonado Tapia CH, Chávez Ruvalcaba F, Moreno García MA (2017) Resiniferatoxin lowers TNF-α, NO and PGE2 in the intestinal phase and the parasite burden in the muscular phase of Trichinella spiralis infection. Parasite Immunology 39(1): 1-14.
- Muñoz Carrillo JL, Muñoz López JL, Muñoz Escobedo JJ, Maldonado Tapia C, Gutiérrez Coronado O, et al. (2017) Therapeutic effects of resiniferatoxin related with immunological responses for intestinal inflammation in Trichinellosis. The Korean Journal of Parasitology 55(6): 587-599.
- Morales Montor Jorge (2010) Descubren en la UNAM que el Tamoxifeno mata al parásito Trichinella spiralis que afecta al ganado. Gaceta UNAM 4249: 12.
- Moreno A, García A, Saldivar S, Reveles R, Muñoz J (2012) Evaluación del Efecto Protector de 3 inmunógenos en modelo experimental murino y cerdo e infectado con Trichinella spiralis. REDVET 13(2): 1-12.
- Reveles HG, Muñoz EJJ, Saldivar ES, Moreno GMA (2000) Efecto de la inmunoterapia sobre larvas infectantes (LI) de Trichinella spiralis implantadas en musculo estriado en modelo experimental. Biotecnología Aplicada 17(2): 126-127.
- Maldonado Tapia C, Reveles Hernández RG, Saldivar Elías S, Muñoz Escobedo JJ, Morales Vallarta M (2007) Evaluación del efecto protector de 2 inmunógenos de Trichinella spiralis en ratas Long Evans con modificación nutricional e infectado con spiralis. Archivos Venezolanos de Farmacología y Terapéutica. 26(2): 110-114.
- Chavez Ruvalcaba F, Chavez Ruvalcaba MI, Hernández Luna CE, Muñoz Escobedo JJ, Muñoz Carrillo JL (2017) Evaluation of anti-Trichinella spiralis obtained by sublingual and conventional immunizations with the 45 kDa protein. Acta Biológica Colombiana 22(2):149-156.
- Crespo JLE, Maldonado TC, Muñoz EJ, Crespo JP, Moreno GA (2018) Implementando la vía sublingual contra Trichinellosis. Editorial Académica Española, p. 1-95.
- Martínez Miranda L, Lara Castro M, Torres García M (2001) Estabilidad de tabletas de rifampicina 300 mg. Rev Cubana Farm 35(1): 18-22.
- Bayona Jaime (2009) Nuevos fármacos antituberculosos. Acta Med Per 26(4): 247-250.
- Hernández Barrera JC, Angarita Merchán M, Prada Quiroga CF (2017) Impacto del uso de antimicrobianos en medicina veterinaria. Rev Cien Agri 14(2): 27-38.
- NOM-062-ZOO-1999. 2001. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Ciudad de México: Diario Oficial de la Federació
- http://www.ibt.unam.mx/ computo/pdfs/bioterio.NOM-062.
- (1957) Armed Forces, Institute of Pathology. Manual of Histologic and Special Staining Techniques. Washington, DC Armed Forces, Institute of Pathology 7: 1-36.
- Towbin HTY, Sthanlin T Gordon (1979) Electrophoretic transfer of proteins from polyacrilamide gels to nitrocelulosa sheets, procedure some application. Proct Nathl Acado sci USA 76(9): 4350.
- Laemmil UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature (London) 227: 680-685.
- Pozio E, Zarlenga D (2019) International Commission on Trichinellosis: recommendations for genotyping Trichinella muscle stage larvae. Food and Waterborne Parasitology 12: 1-9.
- Ewa Bilska Zając, Mirosław Różycki, Katarzyna Grądziel Krukowska, Aneta Bełcik, Iwona Mizak (2020) Diversity of Trichinella species in relation to the host species and geographical location. Veterinary parasitology 279: 1-7.
- Alvin A Gajadhar, Karsten Noeckler, Pascal Boireau, Patrizia Rossi, Brad Scandrett (2019) International Commission on Trichinellosis: Recommendations for quality assurance in digestion testing programs for Trichinella. Food and Waterborne Parasitology 16: 1-20.

 Research Article
Research Article