Abstract
Sponges serve as hosts for diverse organisms and hence are reservoirs of various natural products. The two symbiotic bacteria obtained from the marine sponge Spongia officinalis var ceylonensis were identified as Micrococcus and Planococcus by morphological, biochemical, and molecular methods. The organic extract prepared from these sponge-associated bacteria was subjected to various bioactivity assays. The ethyl acetate extracts from the isolates were found to exhibit antioxidant and antiacetyl cholinesterase activity. These extracts exhibited antiproliferative action against the three cell lines tested with the lowest IC 50 value against the HT-29 cells. The GCMS analysis of these extracts revealed the presence of several bioactive compounds such as eugenol, phenol-2, 4-bis (1phenylethyl), methyl salicylate, 1, 2- benzenedi carboxylic acid esters, octadecanoic acid, squalene, and cannabinol. These bioactive compounds may be contributing to the activity of these symbiotic bacterial extracts and these symbiotic bacteria could serve to meet the supply problem in marine drug development.
Keywords: Marine sponge symbionts; Micrococcus; Planococcus; Spongia officinalis var; Ceylonensis; Bioactivity; Natural Compounds
Introduction
Marine sponges are envisaged as microbial diversity hotspots, as they harbor enormous diversity of microorganisms. Symbiotic microbes have recently been demonstrated as the site of biosynthesis of sponge-derived metabolites with pharmaceutical activity. The diversity of microorganisms inside these evolutionarily oldest animals covers most bacterial phyla. Sponges maintain a unique relationship with the microbes which constitutes up to 40-60% of their biomass [1]. These diverse microbes enter the sponge body through their filter-feeding habit, and some of them are ingested while others reside inside the sponge mesohyl as symbionts. These symbionts play significant roles in the nutrition, health, and chemical defense of sponges, and the symbionts, in turn, benefit from nutrients and protection by the sponge host [2]. Approximately 0.1% of the bacterial species are known until now [3] and approximately 32 bacterial phyla and candidate phyla have been found in sponges [4]. This clearly illustrates the great microbial diversity left unexplored which may serve as a good source of bioactive compounds. The compounds from marine symbiotic microbes have been found to possess varied biological activities as several metabolites with antibacterial, antioxidant, immunosuppressive, antihypertensive, hypocholesterolemic, antiplasmodial, and anticancer activities are reported from them.
The marine sponge, Spongia officinalis var ceylonensis, found exclusively in the Indian Ocean was found to possess significant pharmaceutical property [5,6]. This sponge belongs to the order Dictyoceratida which contributed the greatest number of compounds in the period 2001- 2010 [7]. The symbiotic microbes may be the producers or involved in the production of these metabolites in marine invertebrates. Some bacterial symbionts are host-specific and will be actively involved in the synthesis of speciesspecific compounds. Though the marine sponges are well known for their symbiotic microbial association, sponges of the Indian coast are not much explored beyond their bioactive potential. Here, we try to identify the symbiotic bacteria significantly contributing to the bioactivity of this sponge species.
Methodology
Sponge Collection and Isolation of Sponge-Associated Bacteria
The marine sponge Spongia officinalis var. ceylonensis was collected from the Southwest coast of India at a depth of 8-10 feet and was transferred to the laboratory in sterile plastic bags. The samples were identified with the help of Dr. P.A. Thomas, Retd. Principal Scientist, CMFRI, Vizhinjam. The collected marine sponge sample was washed thoroughly with artificial seawater followed by 70% alcohol. The treated sponge specimen was homogenized with sterilized mortar and pestle and was serially diluted. These serially diluted samples were spread plated and streaked onto agar plates containing the Zobell marine broth medium. The colonies with distinct morphology were re-streaked to obtain pure colonies. Of the 12 bacteria isolated, two isolates - MBTU SOLY1 and MBTU SOBOP1 that showed greater cytotoxicity in brine shrimp lethality assay were chosen for further studies. The selected strains were preserved in 20-30% glycerol at -20ºC.
Morphological, Biochemical and Physiological Characteristics of the Symbiotic Bacteria
The pure colonies of the two selected isolates were carefully observed for identifying their morphology. Standard protocols were used for determining motility, gram staining, and various biochemical characteristics such as catalase and oxidase activities, nitrate reduction, etc. An extensive range of biochemical properties was analyzed by using the VITEX2 system. Physiological characterization of the two strains was done after adjusting the absorbance of the broth cultures to 1OD and various parameters such as different concentrations of NaCl, various temperatures, pH, and varying seawater concentrations were evaluated.
Phylogenetic Identification of the Isolates
The two isolates were identified molecularly by employing the primers 27F (5’ AGAGTTTGATCMTGGCTCAG 3’) and 1492R (5’ AAGGAGGTGWTCCARCC 3’) for the amplification of 16S rRNA. PCR was carried out as described by Chun and Goodfellow [8]. The amplification program consisted of an initial denaturation step of 94ºC for 3 min, and 30 cycles of reaction with each cycle of 94ºC for 30s, 58ºC for 30s, 72ºC for 2min, and a final extension step of 72ºC for 7minutes. PCR product was checked by agarose gel electrophoresis, amplified DNA fragments were observed and sequencing was performed. The unknown bacterium was identified using the Genbank database. The obtained sequences were analyzed using the BLAST tool to get the relative identification of each bacterial species [9] and the culture sequences were deposited in Genbank through NCBI and obtained accession numbers.
Preparation of Microbial Extract
The isolates were inoculated into sterile Zobell marine broth and kept at 25°C for 3 days. Turbidity was seen during the incubation period. 1mL (~4%) of inoculum of three-day-old cultures were transferred to 250mL marine broth and kept at 25°C for 7 days. The cultures were then centrifuged at 10000 rpm for 30 minutes and the supernatant was collected. The collected supernatants were lyophilized and the solvent ethyl acetate was added in the ratio 1:1 to the supernatant taken in a separating funnel and extracted for three days. The extracts were then concentrated by evaporating the solvent in a rotary evaporator. The dried extracts were stored at -20°C.
Screening of Chemical Constituents
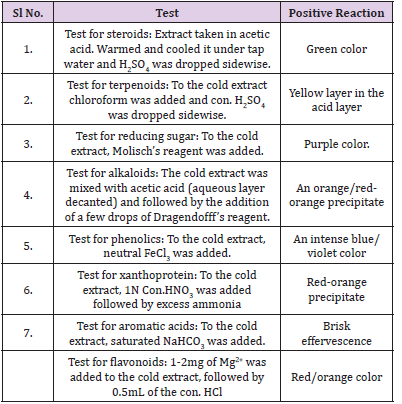
The ethyl acetate extracts of the symbiotic microbial isolates were screened for chemical constituents with slight modifications of the method (Table 1) of Harborne [10].
Antioxidant Activity assay
The organic extracts of the symbiotic microbial extracts were subjected to the following antioxidant assays.
DPPH Radical Scavenging Activity Assay
The stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of the microbial extracts was determined using the method of Mensor et al. [11]. The samples and the reference at differing concentrations of 62.5μg/mL, 125μg/mL, 250μg/mL, 500μg/mL and 1000μg/mL were dissolved in methanol were then mixed with the DPPH solution. The remaining DPPH amount was measured at 517nm using a spectrophotometer. Ascorbic acid was employed as the reference standard. Inhibition of DPPH in percentage was calculated as given below:
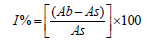
where Ab is the absorbance of the control reaction and As is the absorbance of the extracts.
Nitric Oxide Scavenging Assay
This assay was performed according to the protocol of Rana et al. [12]. 10mM Sodium nitroprusside (2mL) in phosphate buffer saline was incubated with the test compounds at different concentrations for 30 minutes at room temperature. 0.5mL of the incubated solution was then mixed with mL of Griess reagent and the absorbance was measured at 546nm. Ascorbic acid was used as the reference standard. The radical scavenging activity was calculated as per the equation:
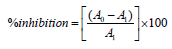
where A0 is the blank and A1 is the test sample.
Acetylcholinesterase Inhibition Assay
The acetylcholinesterase inhibition activity was performed for the extracts of the sponge symbiotic bacteria at varying concentrations according to the method of Ellman et al. [13], with slight modifications. Acetylcholinesterase (AChE) from electric eel (Sigma, USA) was dissolved in 100mM phosphate buffer (pH 7.3) to a final concentration of 500EU/mL. 140μL of the Ellman reagent (5, 5-dithiobis-2-nitrobenzoic acid) in 25 mM phosphate buffer (pH 7.0) is added to each microtiter plate wells followed by the addition of 10μL acetylcholine (ACh) in 1mM final concentration, 20μL of sponge sample, and 50μL of AChE. Deionized (20μL) water was used as controls. Galantamine was used as the reference standard. The time course of the enzymatic reaction was monitored for 12 minutes at 412nm at 25°C.
Antiproliferative Activity
The ethyl acetate extracts of the two isolates were subjected to MTT assay against HT-29 colon cancer cell line, MCF-7 breast cancer cell line, and HeLa cervical cancer cell lines purchased from NCCS Pune. These cells were maintained in Dulbecco’s Modified Eagles Media (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and grown to confluency at 37°C in 5% CO2 in a humidified atmosphere in a CO2 incubator. The trypsinized (500μL of 0.025% Trypsin in PBS or 0.5mM EDTA solution for two minutes) cells were passaged to T flasks under aseptic conditions. The cells were then treated with varying concentrations (6.25μg/mL, 12.5μg/mL, 25μg/mL, 50 μg/mL and 100μg/mL) of the extract from a stock of 1mg/mL. The percentage viability was determined by standard MTT assay after 24 hours of incubation. 30 μL of MTT solution (5mg/mL in PBS) was then added to the treated cells after 1X PBS wash. It was then incubated at 370C for 3-5 hours. MTT was removed by washing with 1X PBS and 200μL of DMSO was added to the culture and incubated for half an hour. Absorbance was read at 540 nm employing DMSO as blank in a microplate reader.
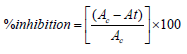
where Ac is OD of Control and At is OD of Test.
Statistical Analysis
The results of the experiments done in triplicates were analyzed by One way ANOVA and were considered statistically significant when p<0.05. Error bars in graphs represent standard error of means per triplicate samples and IC 50 values were calculated in MS Excel. The values were compared by Tukey’s post hoc test and the superscript on the table represents the values that differ significantly (***P<0.0001, **P< 0.001, *P < 0.005).
GC-MS Analysiss
The ethyl acetate extracts of the two isolates were subjected to GCMS analysis. Analyses were carried out on an Agilent GC 7890A MS575C fitted with DB-SMS 30x0.25mmx0.25μm using Helium as the carrier gas. The injective temperature was set at 250°C. The column temperature was initially kept at 40°C for 5 min, then gradually increased to 250°C at a rate of 5°C/min and finally raised to 280°C hold for 10 minutes. The identification of the compounds was done with the help of the NIST library.
Results
Morphological, Biochemical and Physiological Characteristics of the Symbiotic Bacteria
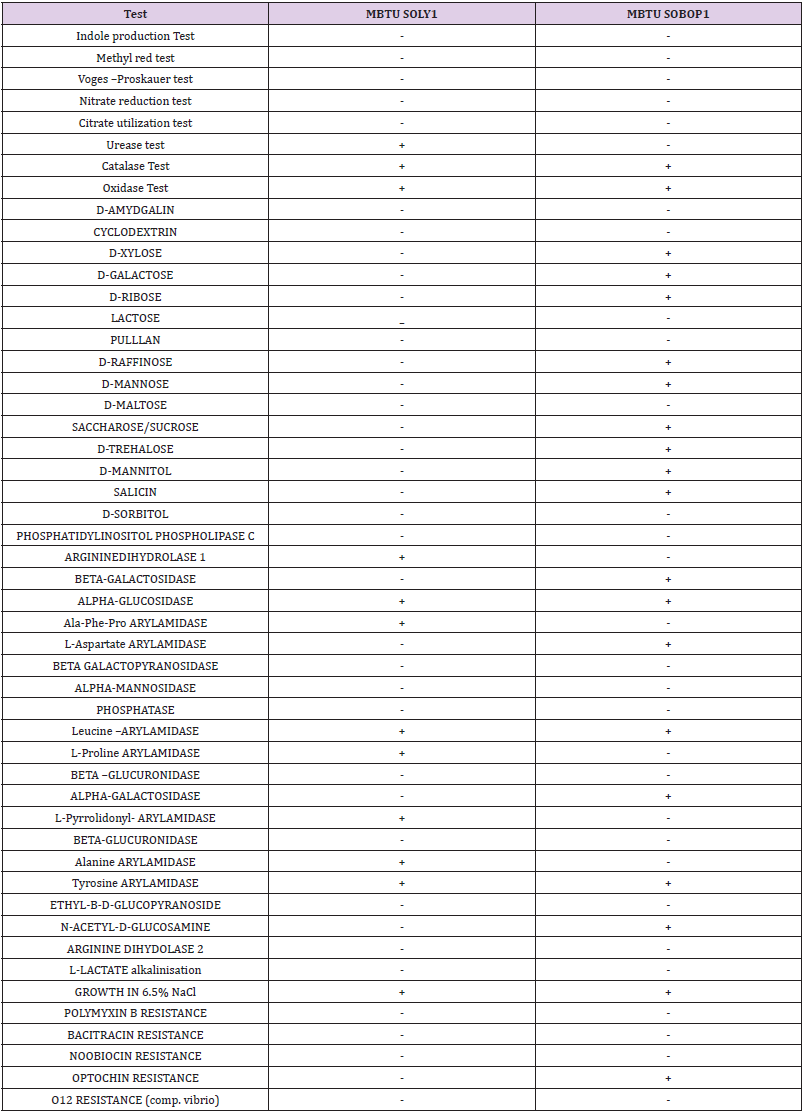
The two isolates- MBTU SOLY1 and MBTU SOBOP1, obtained from the sponge host Spongia officinalis var ceylonensis were identified by observing their culture characteristics, microscopic observation, biochemical identification, and molecular identification. MBTU SOLY1 was a yellow, small, entire, opaque, round, and smooth colony whereas MBTU SOBOP1 was an orange, small, round, entire, flat, and smooth colony. Both the isolates were gram- positive, non-motile cocci. The biochemical characterization of the isolates obtained by both manual and VITEK 2 system is summarized in Table 2. The results indicated that both the isolates were positive for catalase, oxidase, and alpha-glucosidase, possessed arylamidase specific for leucine and tyrosine, and capable of growth in 6.5% NaCl. The isolate MBTU SOLY1 was urease positive, arginine dihydrolase positive (can utilize arginine as a substrate), and possessed arylamidases for Ala-Phe-Pro, L-Proline, L-pyrrolidonyl, and alanine whereas it was incapable of sugar fermentation. The isolate MBTU SOBOP1 was optochin resistant, possessed arylamidase for L-aspartate was positive for alpha-galactosidase and beta- galactosidase, N-acetyl –D- glucosamine, and could ferment the carbohydrates-D- xylose, D-galactose, D-ribose, D-raffinose, D-mannose, saccharose/sucrose, D-trehalose, D- mannitol, salicin, and D-sorbitol.
The physiological characterization of the isolates revealed that the optimum temperature for both the cultures was at 25°C. They were capable of surviving till 55°C which indicates the thermophilic nature of the isolates, but the growth was found to decrease with temperature. The optimum pH was 8 for both the cultures, but the growth of both the isolates got inhibited at a lower pH range. The optimum NaCl concentration was 7% for MBTU SOLY1 and 4% for MBTU SOBOP1 and these isolates could survive up to 30% NaCl concentration. The Zobell medium in 50% seawater and 2% NaCl concentration was found to be the suitable medium for the optimum growth of the selected isolates on monitoring the growth of these isolates in different media such as Zobell media in 50% seawater, Zobell media in 100% seawater, Zobell media in distilled water, Zobell media in 50% seawater, Zobell media in distilled water and 2% NaCl concentration.
Phylogenetic Identification of the Isolates
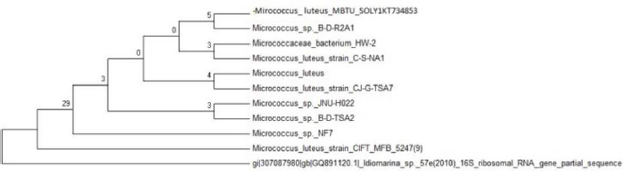
The sequencing results confirmed that MBTU SOLY1 belonged to Micrococcus luteus sp. (Figure 1) and MBTU SOBOP1 belonged to Planococcus sp. (Figure 2). Both the sequences were deposited in Genbank through NCBI and obtained accession numbers as KT734853 and KX236450 respectively.
Screening of Chemical Constituents
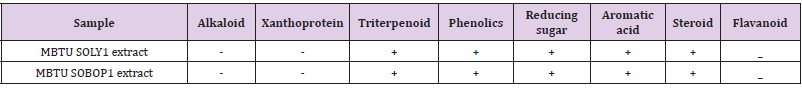
The supernatants of both the isolates were lyophilized and were subjected to extraction using the solvent ethyl acetate. The chemical constituent analysis (Table 3) revealed the presence of similar chemical entities in both the extracts such as triterpenoids, phenolics, reducing sugar, aromatic acid, and steroid. These chemicals are potent bioactive entities that may contribute to the bioactivity of the extracts.
Table 3: Screening of chemical constituents in microbial extracts- ‘+’ indicates the presence and ‘-‘indicates an absence of the chemical constituent in the extract.
Antioxidant Activity
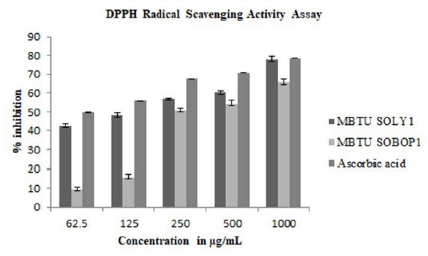
Antioxidants find major application in ailments such as cancer, aging, and atherosclerosis due to the involvement of free radicals in disease development. The extracts of both the isolates exhibited significant antioxidant activity. DPPH radical scavenging activity assay was performed for different concentrations of both the extracts of the two isolates at 62.5μg/mL, 125μg/mL, 250μg/ mL, 500μg/mL, and 1000μg/mL concentrations to test the free radical scavenging ability of these extracts. The DPPH free radical is reduced to yellow diphenylpicrylhydrazine at all the tested concentrations. The extracts exhibited scavenging activity in a concentration-dependent manner. The inhibition percentage ranges from 9.4-80.06% and the extract of MBTU-SOLY1 exhibited greater activity (Figure 3).
Figure 3: DPPH radical scavenging activity of microbial extracts- Error bars in graphs represent standard error of means per triplicate samples.
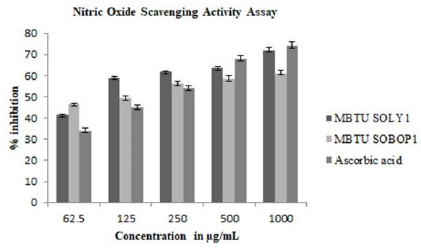
Nitric oxide is a bioregulatory molecule and exerts various physiological effects including blood pressure, neuronal signal transduction, platelet function, antimicrobial and antitumor activity. Increased nitric oxide production is a sign of inflammation and infection causes renal dysfunction and tumor growth. Both the extracts possessed NO scavenging activity and the extract of MBTU SOLY1 exhibited greater activity with an IC 50 value of 78.35μg (Table 4). Both the extracts exhibited elevated nitric oxide scavenging activity in a dose-dependent manner (Figure 4).
Table 4: IC50 values of antioxidant assays- The superscript on the table represents the values that differ significantly (***P<0.0001, **P< 0.001, *P < 0.005).
Acetylcholinesterase Inhibitory Activity
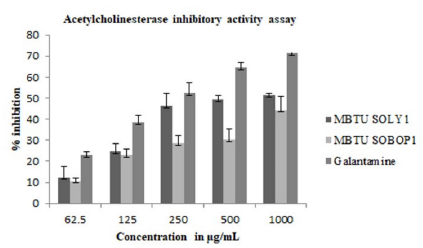
Anticholinesterases cause the accumulation of acetylcholine at their sites of action thus stimulating the parasympathetic nervous system-slowing heart action, lowering blood pressure, increasing secretion, and inducing contraction of smooth muscles. In this study, AChE inhibitory activity (Figure 5) was greater for MBTU SOLY1 extracts with an IC 50 value of 531μg (Table 5).
Table 5: IC50 values of AChE inhibitory activity assay- The superscript on the table represents the values that differ significantly (***P<0.0001, **P< 0.001, *P < 0.005).
Figure 5: Acetylcholinesterase inhibitory activity of microbial extracts- Error bars in graphs represent standard error of means per triplicate samples.
Antiproliferative Activity
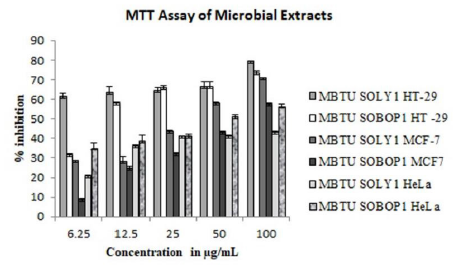
MTT assay for ethyl acetate extracts of microbes was performed against three cancer cell lines- HT-29, MCF-7, and HeLa cell lines. Their activities were concentration-dependent and the viability of the cells decreased with the increasing concentrations of both the extracts. Against all the three tested cell lines, the extract of MBTU SOLY1 was more active (Figure 6 and Table 6). Among these three cell lines, the microbial extracts were more active against the HT- 29 cell line with an inhibition range of 61-79% for MBTU SOLY1 extract and 31-73% for MBTU SOBOP1 extract.
Table 6: IC50 values of MTT assay- The superscript on the table represents the values that differ significantly (***P<0.0001, **P< 0.001, *P < 0.005).
Figure 6: MTT assay of microbial extracts- Error bars in graphs represent standard error of means per triplicate samples.
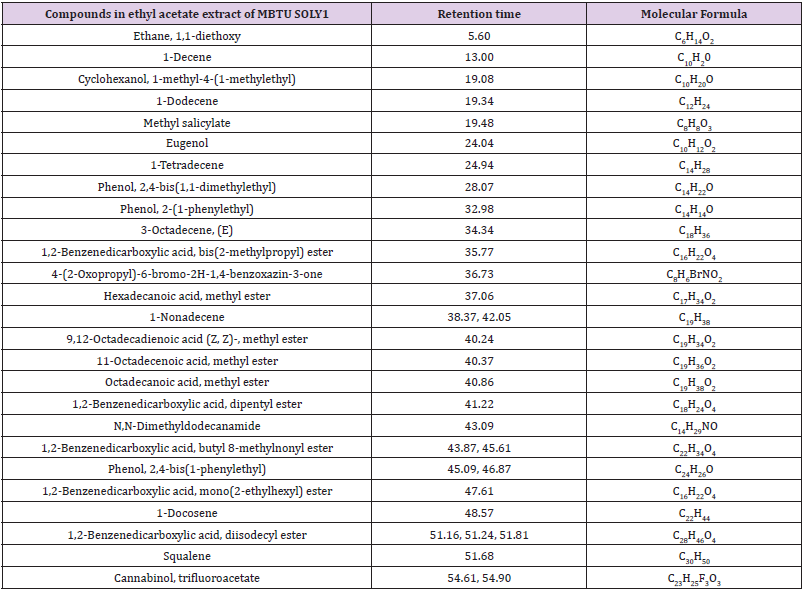
GC-MS Analysis
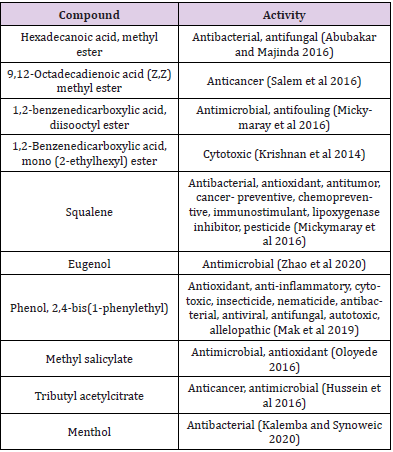
The two bioactive ethyl acetate extracts were analyzed by GCMS. The result indicated that both the extracts contained peaks of the compounds eugenol, phenol-2,4-bis(1phenylethyl), methylsalicylate, 1,2-benzenedicarboxylic acid esters, fatty acid esters, squalene, and cannabinol (Figures 7&8; Tables 7&8). All these bioactive compounds such as phenol-2,4- bis(1phenylethyl), 1,2-benzenedicarboxylic acid esters, and fatty acid esters were also present in the host sponge extract (Supplementary material). These compounds are reported to have antibacterial, antitumor, antioxidant activities (Table 9). This proves the symbiotic association of sponge with these microbes.
Discussion
Marine sponges are well-known hosts for diverse microbes. These microbes produce various secondary metabolites with the prospect of developing into useful drugs. Numerous compounds have been reported from marine sponges and many of them are believed to be the products of symbiotic microbes in them. In this study, the two gram-positive halophiles-MBTU SOLY1 and MBTU SOBOP1 isolated from the marine sponge Spongia officinalis var. ceylonensis were identified as Micrococcus luteus and Planococcus sp. respectively. This is the first report of isolating Micrococcus and Planococcus from the marine sponge Spongia officinalis var. ceylonensis from the Indian coast. The Micrococcus was isolated from various other marine sponges [14-17] and the Planococcus sp. was isolated from the marine sponge, Plakortis simplex [18]. Li [19] reviewed that the Micrococcus sp. has been previously isolated from Hymeniacon perleve, Mycale adherens, and Callyspongia sp. whereas the Planococcus sp. was isolated from Craniella australiensis. The isolate MBTU SOLY1 belonged to Phylum Actinobacteria and the isolate MBTUSOBOP1 belonged to Phylum Firmicutes. Thomas et al [17] have reported that the Phylum Actinobacteria is the most abundant in terms of the distribution of compound production in sponge symbionts which is followed by the Phylum Firmicutes. In an approach to isolate bacteria from Spongia officinalis, Karimi et al [20] revealed that the isolates belonged to the phyla Proteobacteria, Actinobacteria, and Firmicutes.
The chemical screening of organic extracts of the isolates revealed the presence of triterpenoids, phenolics, reducing sugar, aromatic acid, and steroid in them which may be the contributors to the bioactive property of the extracts. Phytochemical characterization of metabolites of ethyl acetate extracts of Pseudomonas sp. from Callyspongia sp. showed that it contained alkaloids, quinones, flavonyl glycosides, and flavonoids [21]. In the same study, the extract from Bacillus sp. from Haliclona sp. was reported to contain carbohydrates, proteins, lipids, alkaloids, quinones, and flavonyl glycosides. The bioactive ethyl acetate extract of Bacillus sp. from the marine sponge Petrosia sp. from Bira Island, Jakarta, Indonesia contained flavonoids, steroids, and tannins [22]. Cita, et al. [23] reported the presence of alkaloids, steroids, and phenols in the ethyl acetate extract of the bacteria from the marine sponge Xestospongia testudinaria. In this study, both the extracts were found to possess significant antioxidant activity. The antioxidant activity of the extracts may be mainly due to the presence of the electron-donating nature of the bioactive chemical constituents present in them. Antioxidant activity of various sponge-associated bacteria was reported by various researchers [24-26]. Kim, et al. [27] evaluated the antioxidant activity of ethyl acetate extracts of 21 different marine bacteria isolated from floats, marine algae, animals, and sponges from Jeju Island, Korea.
Acetylcholinesterase has a role in cell growth, signaling, and tumorigenesis which evokes the potential for anticancer drug development. The ability of the extracts to accumulate acetylcholine in the synapses gives an additional prospect for developing a drug molecule for neurodegenerative disease. The anticholinesterases in clinical use today are mainly for treating neurodegenerative diseases such as Alzheimer’s disease and for augmenting gastric and intestinal contractions and muscular contractions in general and most of them are with side effects. The extracts in the present study exhibited an anticholinesterase effect which holds the prospect for the development of a novel drug candidate. Bacillus subtilis strain from the marine sponge, Fasciospongia cavernosa is reported to have acetylcholinesterase inhibitory activity [28]. The microbial extracts also exhibited concentration-dependent activity against the cancer cell lines and were found to be more active against the HT-29 colon cancer cell line. There are various reports for anticancer activities of extracts of marine sponge-associated bacteria [29,30].
The GCMS analysis of the extracts revealed the presence of several bioactive compounds such as eugenol, phenol-2,4- bis(1phenylethyl), methylsalicylate, 1,2-benzenedicarboxylic acid esters, eugenol, fatty acid esters, squalene, and cannabinol in them. Some of them were common in both the extracts and were also found in the host sponge extract which proves the symbiotic association of these microbes. The compound menthol was only present in the MBTU SOBOP1 extract and not in the MBTU SOLY1 extract. This compound from plant extract is reported to have antibacterial and antioxidant activities by Singh, et al. [31]. There were several reports on utilizing GCMS as a tool for discovering compounds in the extracts of marine bacteria [32-34]. Selvin et al. [35] observed the presence of 1,2 benzenedicarboxylic acid in the GCMS spectrum of ethyl acetate fraction of Nocardiopsis darsonvillei isolated from the sponge Dendrilla nigra. Krishnan, et al. [36] reported that the compound 1,2 benzenedicarboxylic acid, mono (2-ethylhexyl) ester from the marine Streptomyces sp. VITSJK8 exhibited a cytotoxic effect against HepG2 and MCF-7 cell lines. Two non-motile gramnegative bacteria of Verrucomicrobia namely Rubritalea spongiae sp. nov. and Rubritalea tangerine sp. nov. from marine sponges were reported to produce the bioactive triterpene, squalene whose primary source is liver oil from deep-sea sharks and whales [37].
The symbiotic microbes produce these bioactive compounds inside the sponges as it is in the favorable physical environment for the induction of its secondary metabolism. But, the metabolic profile of these microbes changes rapidly once they are devoid of the selective pressure offered by the natural habitat. The successful culture of symbiotic microbes producing bioactive metabolites is of great significance since it will serve as an unlimited source of the respective compound while proving its contribution to the sponge host. Among the two isolates and their extracts, the MBTU SOLY1 ethyl acetate extract was more active in all the bioactivity assays. Though both the tested extracts contained similar chemical constituents, the greater activity of the MBTU SOLY1 extract may be due to the higher concentration of the active principle. The study provides a clear idea with respect to the symbiotic association of the host marine sponge with the bacteria. The significant contribution of the symbionts towards the activity of the host marine sponge enables the economic development of the active compound as it is possible to cultivate the microbe in larger quantities for harvesting the active component in sufficient amounts. Thus, further optimization, bioassay- guided purification, and characterization studies of these isolates will yield potential drug candidates [38- 45].
Acknowledgement
The authors would like to acknowledge the by DST-Inspire Fellowship by the Department of Science and Technology, Ministry of Science and Technology, Govt. of India for supporting the work.
Conflict of Interest
The authors declare that there is no conflict of interest.
Availability of Data and Material
The sequence data were submitted to GenBank through NCBI and obtained accession numbers KT734853 and KX236450. All data generated or analyzed during this study are included in this published article (and supplementary files).
References
- Najafi A, Moradinasab M, Nabipour I (2018) First record of microbiomes of sponges collected from the Persian Gulf, using Tag Pyrosequencing. Front Microbiol.
- Brinkmann CM, Marker A, Kurtböke DI (2017) An Overview on Marine Sponge-Symbiotic Bacteria as Unexhausted Sources for Natural Product Discovery. Diversity 9(4): 40.
- Thomas AT, Rao JV, Subrahmanyam VM, Chandrashekhar HR, Maliyakkal N, et al. (2011) In vitro anticancer activity of microbial isolates from diverse habitats. Braz J Pharm Sci 47(2): 279-287.
- Webster NS, Luter HM, Soo RM, Botte ES, Simister RL, et al. (2013) Same, same but different: symbiotic bacterial associations in GBR sponges. Front. Microbiol.
- Krishnan KAA, and Keerthi, TR (2016) Analyses of Methanol Extracts of Two Marine Sponges, Spongia officinalis ceylonensis and Sigmadocia carnosa from Southwest Coast of India for their Bioactivities. Int. J. Curr. Microbiol. App. Sci 5(2): 722-734.
- Sam W, Nair SCR, Ramachandran R (2017) In vitro pharmacological evaluation of the keratosid sponge Spongia officinalis var ceylonensis (Dendy) for anti-cancer activity. International Journal of Pharmacognosy and Phytochemical Research 9(3): 437-443.
- Mehbub MF, Lei J, Franco C, Zhang W (2014) Marine sponge derived natural products between 2001-2010: Trends and opportunities for discovery of bioactives. Mar Drugs 12(8): 4539-4577.
- Chun J, Goodfellow M (1995) A phylogenetic analysis of the genus Nocardia with 16S rRNA gene sequences. Int J of Syst Bacteriol 45(2): 240-245.
- Zhang Y, Muyers JP, Testa G, Stewart AF (2000) DNA cloning by homologous recombination in Escherichia coli. Nat Biotechnol 18(12): 1314-1317.
- Harborne JB (1998) Phytochemical Methods: A guide to modern techniques of plant analysis, London, Chapman and Hall, 2nd (edn.). pp. 54-84.
- Mensor LL, Menezer FS, Leitao GG, Reis FS, Santos TC, et al. (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res 15(2): 127-130.
- Rana MG, Katbamna RV, Padhya AA, Dudhrejiya AD, Jivani NP, et al. (2010) In vitro antioxidant and free radical scavenging studies of alcoholic extract of Medicago sativa Romanian Journal of Biology Plant Biology 55: 15-22.
- Ellman GL, Courtney KD, Andres VJr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology 7(2): 88-95.
- Bultel-Pounce V, Delitus C, Berge JP, Caceau C (1998) Metabolites from the sponge- associated bacteria Micrococcus luteus. Journal of Marine Biotechnology 6(4): 233-236.
- Matobole RM, vanZyl LJ, Parker-Nance S, Davies-Coleman MT, Trindade M (2017) Antibacterial activities of bacteria isolated from the marine sponges Isodictya compressa and Higginsia bidentifera collected from Algoa Bay, South Africa. Mar. Drugs 15(2): 47.
- Abdelmohsen R, Bayer K, Hentschel U (2014) Diversity, abundance and natural products of marine sponge-associated actinomycetes. Nat Prod. Rep pp. 1-19.
- Thomas TRA, Kavlekar DP, LokaBharathi PA (2010) Marine drugs from sponge-microbe association-a review. Mar. Drugs 8(4): 1417-1468.
- Kaur I, Das AP, Acharya M, Klenk HP, Sree A, et al. (2012) Planococcus plakortidis nov., isolated from the marine sponge Plakortis simplex (Schulze). Int J Syst Evol Microbiol 62(4): 883-889.
- Li Z (2009) Advances in marine microbial symbionts in the China Sea and related pharmaceutical metabolites. Mar. Drugs 7: 113-129.
- Karimi E, Keller-Costa T, Slaby BM, Cox CJ, da Rocha UN, et al. (2019) Genomic blueprints of sponge prokaryote symbiosis are shared by low abundant and cultivatable Alphaproteobacteria. Scientific Reports 9(1999): 1-15.
- Skariyachan S, Rao AG, Patil MR, Saikia B, Bharadwaj Kn V, et al. (2014) Antimicrobial potential of metabolites extracted from bacterial symbionts associated with marine sponges in coastal area of Gulf of Mannar Biosphere, India. Lett Appl Microbiol 58(3): 231-241.
- Prastya ME, Astuti RI, Batubara I, Wahyudi AT (2019) Antioxidant, antiglycation and in vivo antiaging effects of metabolite extracts from marine sponge-associated bacteria. Indian J Pharm Sci 81(2): 344-353.
- Cita YP, Suhermanto A, Radjasa OK, Sudharmono P (2017) Antibacterial activity of marine bacteria isolated from sponge Xestospongia testudinaria from Sorong, Papua. Asian Pac J Trop Biomed 7(5): 450-454.
- Velho-Pereira S, Parvatkar P, Furtado IJ (2015) Evaluation of antioxidant producing potential of halophilic bacterial bionts from marine invertebrates. Indian J Pharm Sci 77(2): 183-189.
- Balakrishnan D, Bibiana AS, Vijayakumar A, Santhosh RS, Dhevendran K, et al. (2015) Antioxidant activity of bacteria associated with the marine sponge Tedania anhelans. Indian Journal of Microbiology 55: 13-18.
- Horta A, Pinteus S, Alves C, Fino N, Silva J, et al. (2014) Antioxidant and antimicrobial potential of the Bifurcaria bifurcata epiphytic bacteria. Mar. Drugs 12(3): 1676-1689.
- Kim HS, Zhang C, Lee JH, Ko JY, Kim EA, et al. (2014) Evaluation of the biological activities of marine bacteria collected from Jeju Island, Korea, and isolation of active compounds from their secondary metabolites. Fisheries and Aquatic Sciences 17(2): 215-222.
- Pandey S, Sree A, Sethi DP, Kumar CG, Kakollu S, et al. (2014) A marine sponge associated strain of Bacillus subtilis and other marine bacteria can produce anticholinesterase compounds. Microbial Cell Factories 13(24): 1-9.
- Priyanto JA, Astuti RI, Nomura J, Wahyudi AT (2017) Bioactive compounds from sponge associated bacteria: anticancer activity and NRPS-PKS gene expression in different carbon sources. American Journal of Biochemistry and Biotechnology 13(4): 148-156.
- Safari WF, Chasanah E, Wahyudi AT (2016) Antibacterial and anticancer activities of marine bacterial extracts and detection of genes for bioactive compounds synthesis. Int J Pharm Sci 8(2): 55-59.
- Singh R, Shushni MAM, Belkheir A (2015) Antibacterial and antioxidant activities of Mentha piperita Arabian Journal of Chemistry 8(3): 322-328.
- Mohan G, Thangappanpillai AKT, Ramasamy B (2016) Antimicrobial activities of secondary metabolites and phylogenetic study of sponge endosymbiotic bacteria, Bacillus at Agatti island, Lakshadweep Archipelago. Biotechnology Reports 11: 44-52.
- Phuong TV, Lam PVH, Diep CN (2018) Bioactive compounds from marine Streptomyces by gas chromatography-mass spectrometry. The Pharmaceutical and Chemical Journal 5(1): 196-203.
- Kiran GS, Priyadharsini S, Sajayan A, Ravindrana A, Selvin J (2018) An antibiotic agent pyrrolo [1,2-α] pyrazine-1,4-dione, hexahydro isolated from a marine bacteria Bacillus tequilensis MSI45 effectively controls multi-drug resistant Staphylococcus aureus. RSC Advances 8 (17837): 1-10.
- Selvin J, Shanmughapriya S, Gandhimathi R, Kiran GS, Ravji TR, et al. (2009) Optimization and production of novel antimicrobial agents from sponge associated marine actinomycetes Nocardiopsis dassonvillei Appl Microbiol Biotechnol 83(3): 435-445.
- Krishnan K, Mani A, Jasmine S (2014) Cytotoxic Activity of Bioactive Compound 1, 2- Benzene Dicarboxylic Acid, Mono 2- Ethylhexyl Ester Extracted from a Marine Derived Streptomyces VITSJK8. Int J Mol Cell Med 3(4): 246-254.
- Yoon J, Matsuo Y, Matsuda S, Adachi K, Kasai H, et al.(2007) Rubritalea spongiae nov. and Rubritalea tangerina sp. nov., two carotenoid- and squalene-producing marine bacteria of the family Verrucomicrobiaceae within the phylum ‘Verrucomicrobia’, isolated from marine animals. International Journal of Systematic and Evolutionary Microbiology 57(10): 2337-2343.
- Abubakar MN, Majinda RRT (2016) GC-MS Analysis and Preliminary Antimicrobial Activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines 3(1): 3.
- Salem MZM, Zayed MZ, Ali HM, Abd El-Kareem MSM (2016) Chemical composition, antioxidant and antibacterial activities of extracts from Schinus, molle wood branch growing in Egypt. J Wood Sci 62: 548-561.
- Mickymaray S, Al Aboody MS, Rath PK, Annamalai P, Nooruddin T (2016) Screening and antibacterial efficacy of selected Indian medicinal plants. Asian Pac J Trop Biomed 6(3): 185-191.
- Zhao F, Wang P, Lucardi RD, Su Z, Li S (2020) Natural Sources and Bioactivities of 2,4- Di-Tert-Butylphenol and Its Analogs. Toxins 12: 35.
- Mak KK, Kamal MB, Ayuba SB, Sakirolla R, Kang YB, et al. (2019) A Comprehensive Review on Eugenol’s Antimicrobial Properties and Industry Applications: A Transformation from Ethnomedicine to Industry. Pharmacogn. Rev 13(25): 1-9.
- Oloyede GK (2016) Toxicity, antimicrobial and antioxidant activities of methyl salicylate dominated essential oils of Laportea aestuans (Gaud). Arabian Journal of Chemistry 9(1): S840-S845.
- Hameed IH (2016) Antimicrobial Activity and Spectral Chemical Analysis of Methanolic Leaves Extract of Adiantum Capillus-Veneris Using GC-MS and FTIR Spectroscopy. International Journal of Pharmacognosy and Phytochemical Research 8(3): 369-385.
- Kalemba D, Synoweic A (2020) Agrobiological Interactions of Essential Oils of Two Menthol mints: Mentha piperita and Mentha arvensis. Molecules 25(1): 59.

 Research Article
Research Article