ABSTRACT
Introduction: Non-palpable breast lesions are findings with not biologically specified importance, which can be responsible for development of cancer. Objective of this study is to present our experience with the diagnosis and the treatment of patients with non-palpable breast lesions at Department of Surgery of Faculty hospital in Nitra.
Material and Methods: The authors present the diagnostic and the results of the treatment of patients with non-palpable breast lesions. They were hospitalized at Department of Surgery in Nitra since January 2014 until July 2017 and we used SNOLL method or wire guided excision under ultrasound control or digital stereotaxic.
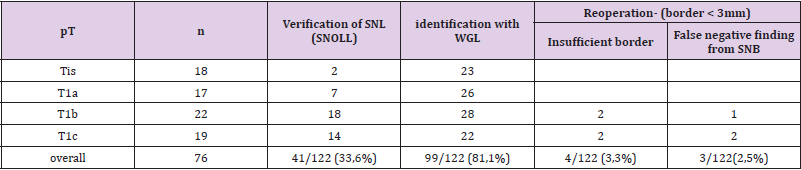
Results: Since January 2014 until July 2017 there were 122 patients diagnosed with non-palpable breast lesions at Department of Surgery of our hospital. 76 (62,3%) of these patients were diagnosed with carcinoma. Wire Guided Localization was performed in 99 (81,1%) patients, sentinel node was found in 41 (33,6%) patients, using of the SNOLL method. From all of the patients the reoperation was conducted in 4 cases (3,3%) for close or positive margin status and in 3 cases (2,5%) for false negative perioperative sentinel biopsy.
Conclusion: The technique combining 99mTc-MAA (albumin-macro aggregate marked by 99Technecium) and nanocoloid is reliable localization method for nonpalpable lesions and sentinel nodes. SNOLL is practical and oncological safe technique of excision subclinical lesion in combination with sentinel biopsy.
Keywords: Breast Cancer; Carcinoma in Situ; Non-Palpable Lesion; WGL; SNOLL Technique
Abbreviations: DCIS: Ductal Carcinoma in Situ; LCIS: Lobular Carcinoma in Situ; PLCIS: Pleomorphic Type of Carcinoma in Situ; HG-DCIS: High Grade Ductal Carcinoma in Situ; WGL: Wire Guided Localization; ROLL: Radioguided Occult Lesion Localization; SNOLL: Sentinel Occult Lesion Localization; 99mTc-MAA: Technetium 99mTc Macro Aggregated Albumin; SLN: Sentinel Lymphatic Node; cm: Centimetre; mm: Millimetre; nm: Nanometre; ml: Millilitre; nM: Nanomolar; MBq- Megabecquerels; cm3: Cubic Centimetre
Introduction
With the notion premalignant non-palpable lesions, we understand spectrum of morphological changes in the tissue of breast. These changes are risk factor for the formation of cancer. These changes are benign, but they are more associated with the cancer with the contrast of another clear benign lesions. We call them high res or precancer lesions. These findings are unclear biological behaviour, cells show malign architectural features and the proliferation is different than the normal regulation mechanism of organism. What is very important, they have no invasion ability or ability to create metastasis. These lesions threaten patients with formation of cancer, but the extent of risk is necessary to connect with pre-existing individual risk factors of every patient [1,2]. At the last decades, the incidence of non-palpable lesions of breast is increasing, because of mammography and another exact imaging methods [3-5]. As a result of this fact, there is the decrease of number of neoplasions and reduction of their spreading into the axillar lymphatic nodes [6,7]. Using of mammographic screening improvement of its sensitivity increase capture ratio of subclinical lesions. Ratio of non-palpable lesions in the time of diagnosis is 25-30% in countries with function screening programme [7]. With increasing diagnostic of non-palpable lesions at the early stadium, correct and complex treatment process is more important. Successful intraoperative localization of non-palpable lesion is necessary for surgeons because of complete excision during the one intervention without extensive excision of healthy tissue.
Histopathological Types of Carcinomas in Situ
There are two types of preinvasive carcinomas, which are different of their features and clinical meaning.
a) Ductal Carcinoma In Situ (DCIS) is the most common type of non-invasive breast cancer. It is straight precursor of invasive ductal carcinoma. During the months and years, it can progress into this malign form (50%).The best treatment method is surgical operation. DCIS starts and spreads inside the milk ducts and has inclination for local relapse. Approximately half of cases of local relapse is presented as invasive carcinoma. (Figures 1 & 2).
Figure 1: Identification of 325 co-expressed DEGs from three microarray data sets (GSE4619, GSE19429 and GSE58831) using R software (version 3.6.2). Different data sets are represented by different color areas. The co-expressed DEGs are indicated by the cross areas.
b) Lobular Carcinoma In Situ (LCIS) is an area (or areas) of abnormal cell growth that increases a person’s risk of developing invasive breast cancer later on in life-both form ductal and lobular. Higher risk of incidence is not only in the place of LCIS but in every area of both breasts. Treatment strategy is not only operative, but also in primary or secondary prevention. Some findings report, that subgroup with atypical nucleuses pleomorphic type (PLCIS), could be straight cancer precursor [8].
Character of growing and spreading is important for treatment and prognosis of DCIS. Faverly´s study of mastectomy preparations shows that DCIS is in 90% spreading single centre (lesion of one quadrant / distance between two focuses >4cm) but can grow also multifaceted (lesions in more separated focus in one quadrant) and discontinued-especially low-grade lesions. Multicentre growth was detected in one case of 60, in 30 cases (50%) was reported discontinued growth. These lesions formed more than 70% low grade carcinomas. Opposite of this, 90% of HG-DCIS growth typical continues [9,10]. Reliable prognostic and predictive markers of DCIS are missing. That’s why the information’s on molecular-genetic level and histopathological parameters are important prognostic meaning of regional relapse. For the management of DCIS grading of lesions is also very important. Precisely histological examining is necessary not only for determination of diagnosis, but also for treatment [11,12].
Material and Methods
Diagnostics and Treatment
Imaging methods have decisive meaning in diagnostics of small non-palpable and clinical silent carcinomas. The method of choice is mammography. Mammography does not serve only for diagnosis of lesions in the breast, but it is useful in intervention operation. Advantage of mammography is ability to find out microcalcifications, which follow carcinoma in 30%. With systematic mammography it is possible to decrease mortality (Figure 3) [13]. Today the core-cut biopsy is gold standard in preoperative diagnostics of non-palpable lesions of breast. During the core-cut biopsy roller of tissue is taken without damage of architect of tissue. It is using special needle, which is attached to mechanic target unit. Suitable calibre of needle is 14G and 16G. By biopsy of clear focus, it is necessary to take 3-4 samples, but by biopsy of unclear focus it is important to take 5-10 samples. [8] Today, there are many methods of preoperative localization of breast lesions. They have to fulfil some basic conditions. Golden standard is localization of nonpalpable lesion with wire (WGL- wire guided localization) (Figures 4 & 5). This technique was introduced by Dodd in 1965. Another modification was technical or shape character of wire (Frank, Homer, Kopans) [14]. By European guidelines for quality of screening and diagnostic of breast cancer the peak of wire must be in maximal 10mm distance from the centre of lesions in minimal of 90% of patients. Excision must be successful more than in 90% of patients. Other conditions are minimum risk of dislocation during localization and surgery and easy identification during surgery.
We realize perioperative identification and extirpation of nonpalpable lesions or suspected microcalcifications:
a) Visual with wire guided localization (Figures 6 & 7)
b) Gamma probe detector (Figures 8 & 9).
Figure 6: Identification of 325 co-expressed DEGs from three microarray data sets (GSE4619, GSE19429 and GSE58831) using R software (version 3.6.2). Different data sets are represented by different color areas. The co-expressed DEGs are indicated by the cross areas.
In 1998 in Europe oncological institute, Luini introduced alternative method of localization of nonpalpable lesion with the name ROLL (radioguided occult lesion localization) [14]. Principle of this technique is based on localization of nonpalpable lesion by radiopharmaceutical, which is fixed on carrier with high molecular weight. Localization is conducted by ultrasonographical navigation, digital stereotaxy or magnetic resonance imaging. By patients with nonpalpable lesion of breast evaluation of axillar lymphatic node is one of the most important factors, so the biopsy of sentinel node is very necessary [15-19]. Combination of ROLL with biopsy of sentinel node is called SNOLL (sentinel occult lesion localization) and it was introduced by deCicco in 2002 [20].
ROLL (radioguided occult lesion localisation) – principal of this technique was derived by technique of sentinel node
a) It is based on localization of small, deep and nonpalpable lesions of breast with measure of 1-10mm with radiopharmaceutical, which are more difficult to be detected by wire.
b) Localization is performed under ultrasonography navigation, digital stereotaxy or magnetic resonance imaging [21,22].
It is used 99mTc-MAA (Technetium 99mTc macro aggregated albumin), which is applicated in volume of 0,2ml with very low activity. Intratumor application in local anaesthesia is done under ultrasonography navigation, digital stereotaxy or mammography control. Indication is exact localization of small deep nonpalpable lesions of breast under 10mm before surgery. Pregnancy and lactation are contraindications. The first phase of examination takes 15-30minutes and radiation load is minimal.
Benefits of ROLL/SNOLL method:
a) Method is more precise than the localization with wire, especially in compact mammary gland.
b) Average time of operation is reduced.
c) Lesions are in the resected tissue placed more in centre, they have wider resection border by the smaller volume of extirpation tissue.
d) Increase in percents of nonpathological resection border.
e) Decrease in percents of require reoperations.
f) Diminish of operations wound and it is less traumatic [23,24].
In our clinic, we proceed according to this two-days protocol:
a) First phase: Indication-Indication of the lesion by 99mTc- MAA, which is collected right in tumor without another extratumor spreading. Examination is possible to accompany with radionuclide detection of sentinel lymphatic node SNOLL = ROLL + SLN
b) Second Phase: perioperative localization- Surgeons by the handy gamma probe localize the place with maximum radioactivity on the surface of the breast. Measurement of impulse of extirpation lesion and its border in operation field is necessary for information about complete extirpation.
Imaging of sentinel node is done by two-days protocol by application of nanonuclide with molecular weight 100 – 600 nM and 99mTc subdermal periareolar in dose 60 MBq patent blau (Figure 5). Control scintigraphy confirmed local and number of hot nodes. Localization of nonpalpable lesion was done day before surgery by the application of Technetium 99mTc macro aggregated albumin with molecular weight 10–100 μm in radioactive dose 20 MBq. Under the USG control, respectively under digital stereotaxy. Marking of lesions were done by specialists from Izotopcentrum. 94% of lesions were marked in distance from 10mm from the centre of lesion. Nonpalpable lesion and sentinel node were detected by gamma probe during the surgery. Operating preparation was subjected RTG examination for the evidence of lesion and wide of healthy resection border. (Figure 8). In case of the fact, that the safety rim was under 10mm, the perioperative resection was done. Reoperation was conducted only if the safety rim was less than 3mm in correlation of histopathological examination.
Results
In Surgery department of Nitra´s hospital we operated from January 2014 to July 2017 122 patients with nonpalpable lesions of breast. By 76 patients (62,3%) the carcinoma was confirmed . Average age of patients in our group was 63,5. Carcinoma was confirmed before surgery by 42 patients ((34,4%)) by core-cut biopsy and by 34 patients (27,9%) the carcinoma was confirmed during the perioperative refrigeration. By 65 patients (53,3%) after imaging nonpalpable lesions core-cut biopsy was done (under the USG navigation and mammography). Image lesions were smaller than 1cm or the cluster of microcalcifications was detected. After the results of core-cut biopsy the carcinoma was confirmed by 42 patients (34,4%) before surgery, by 23 patients (18.9%) only benign lesion was detected). Localization of nonpalpable lesion were done by 99 patients (81,1%) with identification wire. Altogether with localization of lesion with WGL we marked lesions with radiopharmaceutical by 87 patients (71.3%). By 12 patients (9,8%) we applicated patent blau for visualisation. We done lymphoscintigraphy by every patient, who had suspicion of carcinoma-it means by 99 patients (81.1%). At the beginning of surgery, we extirpated suspect lesion and after this, the tissue was subjected RTG because of presence of clusters of microcalcifications. After this, the histopathological examination was done. In case of safety rim under 3mm in correlation of histopathological examination, reresection positive by 6 patients (4,9%) and by 35 patients (28,37%) it was negative.
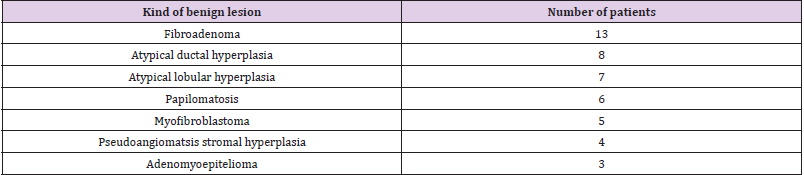
Average amount of eliminated sentinel nodes by one surgery was 2,6. After the confirmation of carcinoma from perioperative refrigeration, we indicated SNOLL by 41 patients (33,6%), Sentinel node was Nonpalpable lesions were extirpated by 99 patients (81,1%). If the result from core-cut biopsy was benign lesion, we did not realize any surgery. We did reoperation by 7 patient s (5,7%). Four times (3,3%) it was because of insufficient border and three times (2,5%) because of false negative result from perioperative histology of sentinel node. Correlation of lesion´s size in histopathological examination to number of reoperation is in Table 1. Schematic figuration of results is possible to view in graph (Figure 10). Benign lesions we noticed in age of 18-65 by 37,7% patients from all group. Malign lesions were characteristic for later incidence at the age of 51-80 by 62,3%patients. Occult, nonpalpable lesions, benign also malign are characterised relative short anamnesis until the surgery. Malign lesions were presented until size of 10mm in 94%. Benign lesions were described with connection of microcalcifications in 55,8%. Extirpation of benign lesions in important in discover of risk group of patients (see Table 2). It is important to do dispensary of these patients in specialized outpatients’ department. We found CIS by 14.8% patients in our group. CIS together with T1 stadium of carcinoma presented 46,05% from all malign cases (76=100%).
Discussion
Examination of asymptomatic women increase capture of nonpalpable lesions of breast. Identification with guide wire remains standard for finding nonpalpable lesions in many workplaces in Europe despite of its deficiencies. These are for example: difficulty for coordination of more specialists in delimited time, cumulation of stress factors because of more invasive processes in surgery day, vaso-vagal syncope during the localization, dislocation, migration, transection of wire, termic damage of skin during surgery, injury of surgeon or pathologist by processing of tissue [25-30]. In 1998 Luini introduced new method with the name ROLL (radioguided occult lesion localization). Principle of this technique is based on localization of sentinel node by radiopharmaceutical, which is fixed on carrier with high molecular weight. Some randomized studies were conducted with this method. De Cicco in group of 227 patients combinated two kinds of radiocarriers with position variations of application of radiopharmac. They have found that the most optimal for imaging of tumor is intratumor application of Technetium 99mTc macro aggregated albumin with combination of subdermal periareolar application of nanonuclid Technetium 99mTc for imaging of sentinel node [20]. Verification of node was demonstrated by 99% of patients. Simonett and Monti have publicized the most extensive study of 959 patients with carcinoma of breast with using of SNOLL.
Study was focused for verification of sentinel node and oncology radicality with using SNOLL [25]. Localization of node was demonstrated in 99,6% of patients, while negative borders were found in 91,9%. Negative border was presented by safety rim > 10 mm. There were presented some studies with intratumor application of nanonuclid for simultaneously localisation tumor and sentinel node [23]. Moreno has randomized group of 120 patients into 2 groups [19]: ROLL a WGL. He identified safety of resection border, cosmetic effect and measure of postoperative pain in first postoperative day. He definited safety borders 10mm for invasive carcinoma and 5mm for DCIS. Both methods safely localized nonpalpable lesion. Volume of excised tissue was in average smaller in group of SNOLL (8,7 cm3 vs. 23,15 cm3 for WGL). In ROLL group statistically significant higher number of clear borders (p < 0,05) was observed, better aesthetic results and less postoperative pain, what was resulted in shorter hospitalisation. There is a new method for detection of sentinel node which is called Sentimag. The Sentimag instrument uses the principle of magnetic susceptometry and generates an alternating magnetic field which transiently magnetises the iron oxide particles in Sienna+. The tiny magnetic signature generated by the Sienna+® particles is then detected by the Sentimag probe. Sienna+ is a dark brown aqueous suspension of organically coated superparamagnetic iron oxide particles. It is injected subcutaneously where the natural physical action of the lymphatic system filters out the particles, enabling sentinel nodes to be located using the Sentimag [26].
One of the benefits of Sienna+ is that this substance is not radioactive. Therefore, it can be used in outpatients’ department or in surgery department without connection with nuclear medicine. There is no need of protection against radioactivity or work with radioactive waste. We can detect substance percutaneous and nodes are brown coloured, what helps by perioperative identification. Indicator in lymphatic node does not underlie quick degradation and is not quickly transporting from node so it is possible to application it with long period before surgery. It can be potentially used by detection of lymphatic nodes by another relevant diagnosis [27]. By concussions of overview studies, radiopharmacological navigated localization obtained many supporters. The reasons are a smaller number of reoperations and better cosmetic effect. Popularity of this method is enhanced with its combination of biopsy of sentinel node. Incidence of CIS dos does not reach 2% in long time period in Slovakia, in screening programmes its incidence raised [28]. Identification of nonpalpable lesions and microcalcifications before surgery is very important by breast carcinoma because early identification requires minimal surgery and minimal multimodal therapy. We can expect minimal incidence of local relapses, higher survival of patients and decrease of mortality. Good cooperation between radiologist, surgeon a pathologist is the guarantee of quality of diagnostic and therapy. We expect more significant effect in survival of patients with nonpalpable lesions of mammal gland (CIS, tumor in T1a) by using MRI with contrast substance and follow-up mark by guide wire before surgery. SNOLL is very practical modification of ROLL method in practise. With combination of sentinel node detection, it is very useful and oncological safety technique of excision of subclinical lesion [31,32].
Conclusion
In pursuance of our study´s results we can state that detection of sentinel node by using 99mTc-MAA presents reliable method for localization of nonpalpable lesions and sentinel node. SNOLL with combination of sentinel node detection is practical and oncological safety technique of excision of subclinical lesion. Alternative method of localization of sentinel node is method Senti-Mag. Benefit of this method is that substance Sienna+ is not radioactive. Examination of asymptomatic women increases number of nonpalpable malign lesions of breast. Identification of nonpalpable lesion with guide wire still golden standard for its verification in many workplaces in Europe.
References
- Shumilov E, Flach J, Kohlmann A, Banz Y, Bonadies N, et al. (2018) Current status and trends in the diagnostics of AML and MDS. Blood Reviews 32(6): 508-519.
- Mufti GJ, McLornan DP, van de Loosdrecht AA, Germing U, Hasserjian RP (2018) Diagnostic algorithm for lower-risk myelodysplastic syndromes. Leukemia 32(8): 1679-1696.
- Chihara D, Ito H, Katanoda K, Shibata A, Matsuda T, et al. (2014) Incidence of myelodysplastic syndrome in Japan. J Epidemiol 24(6): 469-473.
- Dinmohamed AG, Visser O, van Norden Y, Huijgens PC, Sonneveld P, et al. (2014) Trends in incidence, initial treatment and survival of myelodysplastic syndromes: a population‐based study of 5144 patients diagnosed in the Netherlands from 2001 to 2010. Eur J Cancer 50(5): 1004-1012.
- Cogle CR (2015) Incidence and burden of the myelodysplastic syndromes. Curr Hematol Malig Rep 10(3): 272-281.
- Zeidan AM, Shallis RM, Wang R, Davidoff A, Ma X (2019) Epidemiology of myelodysplastic syndromes: Why characterizing the beast is a prerequisite to taming it. Blood Rev 34: 1-15.
- Ye X, Chen D, Zheng Y, Wu C, Zhu X, et al. (2019) The incidence, risk factors, and survival of acute myeloid leukemia secondary to myelodysplastic syndrome: A population-based study. Hematological Oncology 37(4): 438-446.
- Quackenbush J (2006) Microarray Analysis and Tumor Classification. N Engl J Med 354(23): 2463-2472.
- Gusnanto A, Calza S, Pawitan Y (2007) Identification of differentially expressed genes and false discovery rate in microarray studies. Curr Opin Lipidol 18(2): 187-193.
- Pellagatti A, Cazzola M, Giagounidis AN, Malcovati L, Porta MGD, et al. (2006) Gene expression profiles of CD34+ cells in myelodysplastic syndromes: involvement of interferon-stimulated genes and correlation to FAB subtype and karyotype. Blood 108(1): 337-345.
- Pellagatti A, Cazzola M, Giagounidis A, Perry J, Malcovati L, et al. (2010) Deregulated gene expression pathways in myelodysplastic syndrome hematopoietic stem cells. Leukemia 24(4): 756-764.
- Gerstung M, Pellagatti A, Malcovati L, Giagounidis A, Porta MGD, et al. (2015) Combining gene mutation with gene expression data improves outcome prediction in myelodysplastic syndromes. Nat Commun 6: 5901.
- Kim M, Hwang S, Park K, Kim SY, Lee YK, et al. (2015) Increased expression of interferon signaling genes in the bone marrow microenvironment of myelodysplastic syndromes. PLoS One 10(3): e0120602.
- Szikszai K, Krejcik Z, Klema J, Loudova N, Hrustincova A, et al. (2020) LncRNA Profiling Reveals That the Deregulation of H19, WT1-AS, TCL6, and LEF1-AS1 Is Associated with Higher-Risk Myelodysplastic Syndrome. Cancers (Basel) 12(10): 2726.
- Chen An T, Yi Ju C, Chen JJ (2003) Testing for differentially expressed genes with microarray data. Nucleic Acids Res 31(9): e52.
- Phipson B, Lee S, Majewski IJ, Alexander WS, Smyth GK (2016) Robust hyperparameter estimation protects against hypervariable genes and improves power to detect differential expression. Ann Appl Stat 10(2): 946-963.
- Smyth GK (2005) Limma: linear models for microarray data. Bioinformatics and computational biology solutions using R and Bioconductor. New York: Springer, pp. 397-420.
- Yu G, Wang LG, Han Y, He QY (2012) clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16(5): 284-287.
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, et al. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102(43): 15545-15550.
- Powers RK, Goodspeed A, Pielke Lombardo H, Tan AC, Costello JC (2018) GSEA-InContext: identifying novel and common patterns in expression experiments. Bioinformatics 34(13): i555-i564.
- Tang Z, Li C, Kang B, Gao G, Li C, et al. (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45(W1): W98-W102.
- Bejar R, Stevenson K, Abdel Wahab O, Galili N, Nilsson B, et al. (2011) Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med 364(26): 2496-506.
- Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, et al. (2013) Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 122(22): 3616-3627.
- Shallis RM, Ahmad R, Zeidan AM (2018) The genetic and molecular pathogenesis of myelodysplastic syndromes. Eur J Haematol 101(3): 260-271.
- Hosono N (2019) Genetic abnormalities and pathophysiology of MDS. Int J Clin Oncol 24(8): 885-892.
- Boddu P, Kantarjian H, Garcia Manero G, Allison J, Sharma P, et al. (2018) The emerging role of immune checkpoint based approaches in AML and MDS. Leuk Lymphoma 59(4): 790-802.
- Sánchez JMH, Lumbreras E, Díez Campelo M, González T, López DA, et al. (2020) Ana Eugenia Rodríguez Vicente. Genome-wide transcriptomics leads to the identification of deregulated genes after deferasirox therapy in low-risk MDS patients. Pharmacogenomics J 20(5): 664-671.
- Huang HH, Chen FY, Chou WC, Hou HA, Ko BS, et al. (2019) Long non-coding RNA HOXB-AS3 promotes myeloid cell proliferation, and its higher expression is an adverse prognostic marker in patients with acute myeloid leukemia and myelodysplastic syndrome. BMC Cancer 19(1): 617.
- Tang S, Jing H, Huang Z, Huang T, Lin S, et al. (2020) Identification of key candidate genes in neuropathic pain by integrated bioinformatic analysis. J Cell Biochem 121(2): 1635-1648.
- Zhang YJ, Sun YZ, Gao XH, Qi RQ (2019) Integrated bioinformatic analysis of differentially expressed genes and signaling pathways in plaque psoriasis. Mol Med Rep 20(1): 225-235.
- Zhong X, Huang G, Ma Q, Liao H, Liu C, et al. (2019) Identification of crucial miRNAs and genes in esophageal squamous cell carcinoma by miRNA-mRNA integrated analysis. Medicine (Baltimore) 98(27): e16269.
- Yoyen Ermis D, Tunali G, Tavukcuoglu E, Horzum U, Ozkazanc D, et al. (2019) Myeloid maturation potentiates STAT3-mediated atypical IFN-γ signaling and upregulation of PD-1 ligands in AML and MDS. Sci Rep 9(1): 11697.
- Wang H, Zhang TT, Qi JQ, Chu TT, Miao M, et al. (2019) Incidence, risk factors, and clinical significance of Epstein-Barr virus reactivation in myelodysplastic syndrome after allogeneic haematopoietic stem cell transplantation. Ann Hematol 98(4): 987-996.
- Pollyea DA, Hedin BR, O'Connor BP, Alper S (2018) Monocyte function in patients with myelodysplastic syndrome. J Leukoc Biol 104(3): 641-647.
- Le Y (2019) Screening and identification of key candidate genes and pathways in myelodysplastic syndrome by bioinformatic analysis. PeerJ 7: e8162.
- Tian WL, Guo R, Wang F, Jiang ZX, Tang P, et al. (2018) The IRF9-SIRT1-P53 axis is involved in the growth of human acute myeloid leukemia. Exp Cell Res 365(2): 185-193.
- Pidugu VK, Pidugu HB, Wu MM, Liu CJ, Lee TC (2019) Emerging Functions of Human IFIT Proteins in Cancer. Front Mol Biosci 6: 148.
- Liu Y, Lu R, Cui W, Pang Y, Liu C, et al. (2020) High IFITM3 expression predicts adverse prognosis in acute myeloid leukemia. Cancer Gene Ther 27(1-2): 38-44.

 Research Article
Research Article