Abstract
An application of mycorrhiza has a role in achieving the goal of food security. The establishment of the mycorrhiza in soil and their pre-adaptation steps affect food for the growing billions. Nowadays, the use of arbuscular mycorrhizal fungi (AMF) in the agricultural field as biofertilizer is on the rise. Zinc (Zn) is one of the important elements for crop growth and development and possesses negative interaction with excess applied phosphorous (P). An estimation of 50% of the world’s cereal growing soils is considered to be low in plant-available Zn. More than 33% of the world population is affected by Zn deficiency. The two different pathways for the uptake of P and Zn from the soil are mycorrhizal pathway uptake (MPU) and direct pathway uptake (DPU). The contribution of Zn by MPU and DPU varied in small quantities (i.e., in μg). In this regard, 24% of the Zn has transferred through the MPU pathway. This type of result has important implications in plants grown with low Zn concentration and high phosphorous application. Under high soil Zn concentration, there is little influence of MPU over DPU. MPU is active when soil Zn supply is low. An important repercussion for crop growing in Zn deficient soil. The relative contribution by the MPU was reduced in huge amounts while the activity of DPU increased with increasing soil Zn supply. Furthermore, a comparative study between mycorrhizal and non-mycorrhizal plants cannot tell us about the activity and interplay between MPU and DPU. An independent study is required to draw valid conclusions. Therefore, it can be concluded that the interplay between DPU and MPU of Zn and P is highly complex and due attention has to be paid for future research. Furthermore, the balanced use of MPU for the soil Zn and P is highly recommended.
Keywords: Arbuscular Mycorrhizal Fungi; Mycorrhizal Pathway Uptake; Direct Pathway Uptake; Zinc; Phosphorous
Mycorrhiza
Mycorrhiza has several functions in a natural ecosystem viz: plant growth, nutrient absorption, disease prevention, stress tolerance, assimilation of photosynthates, and many more. There are other organisms than mycorrhiza in the rhizosphere like bacteria and actinomycetes. These types of organisms are beneficial for nutrient solubilization and release. Among all the various types of microorganisms in the rhizosphere arbuscular mycorrhizal fungi (AMF) help in the uptake of nutrients from plant roots. The application of AMF in the agricultural field as a biofertilizer is on the rise. The use of AMF is reported to have indirect benefits in the plants by reducing herbivore visitation rates and reducing the impact of herbivore damage. Firstly, infection by AMF appears to increase plant defenses against generalists for example chewing herbivores. Secondly, AMF promotes indirect defenses like a release of volatiles to attract herbivore enemies and defend against piercing and sucking insects [1]. Besides in this study, we are dealing with how the two ways of nutrient uptake pathway that differed in phosphorous (P) acquisition and zinc (Zn) nutrition. The two different pathways for the uptake of Zn and Pare direct pathway uptake (DPU) and mycorrhizal pathway uptake (MPU).
Mycorrhizal Pathway Uptake (MPU) vs Direct Pathway Uptake (DPU)
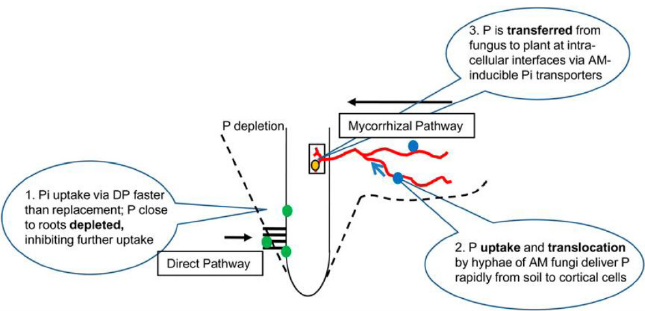
Two nutrient uptake pathways are functioning in the arbuscular mycorrhizal plant. First is the direct pathway uptake (DPU) through the root epidermis and the second is through mycorrhizal pathway uptake (MPU). In a study with maize, the greatest MPU value of 21.7μg was observed. The highest relative contribution of the MPU was up to 24.2% in Zn concentration treatment Zn 1 μg g-1 [2]. Likewise, with P uptake plant Zn uptake is highly determined by the host plant species [3]. So, it is important to quantify the MPU for Zn in different crop species. To the best of our knowledge till now there are no studies that have measured the contribution of the Zn to the edible portion of any crop species. The study aforementioned had the following specific aims. (a) To quantify the wheat and barley grain in MPU to Zn. (b) to investigate whether the contribution to plant Zn via the MPU differed under a wide range of soil conditions. (c) To study the effects of AMF inoculation on plant yield and Zn concentration in a crop like bread wheat and barley. There is not a significant difference between the MPU activity in low and medium Zn but it has significantly differed from the high Zn [2]. Likewise, the Zn delivered by MPU to the straw and total plant did not change in low Zn and medium Zn respectively. But this value is significantly higher at high Zn. Concerning the concentration of the grain, straw, and total plant Zn, MPU delivered the same amount of Zn at low Zn and medium Zn levels. The amount of Zn delivered by MPU to the grain, straw, and total plant are 21.4, 51.6, and 73 μg plant-1 respectively at low, medium, and high Zn concentration [2]. Mycorrhizal pathway of Zn uptake (MPU) is helpful to 24% of the shoot uptake of Zn at the lowest soil Zn application. A decrease in the Zn uptake is observed significantly to 8% when soil Zn concentration is high. Increasing the concentration of soil Zn decreases the uptake of Zn through MPU while there is an increment of Zn uptake at low soil Zn concentration [4] Figure 1 [5-7].
An estimation of 50% of the world’s cereal growing soils is considered to be low in plant-available Zn [8]. More than 33% of the world population is affected by Zn deficiency [9]. Besides this Zn level can reach toxicity to plants. So basic understating of different factors in Zn regulation is important [4]. Under low Zn concentrations plant with mycorrhiza has a higher concentration compared to non-mycorrhizal plants [10]. In toxic level of soil Zn concentration in plants, mycorrhiza protects the plant from excess Zn concentration in comparison to a non-mycorrhizal plant grown in the same soil [11]. This is how the mycorrhiza plays an important role in modulating Zn acquisition under a broad range of soil Zn concentrations. The contribution of Zn by MPU and DPU is varied in small quantity i.e., in μg [4]. It is still unknown about the amount of Zn uptake by MPU [4]. This is how a study of the function of MPU in response to Zn acquisition is of high priority. There is a significant proportion of plant Zn can be delivered via the MPU at low concentrations of the Zn in soil. But increase in the soil Zn level reduction of the uptake is possible through MPU. And it has been hypothesized that P-induced Zn deficiency can be alleviated with MPU of Zn. There is mycorrhizal colonization at 76R genotype with the application of 0, 20, and 50 mg Kg-1 level of Zn. In the genotypes 76R and rmc of the tomato a significant difference in root and shoot Zn concentration are recorded. But P concentration did not differ as the application of Zn increased from 0, 20, and 50 mg Kg-1 respectively [4]. Up to 24.2% of the total Zn entered in the shoots of the 76R genotypes is delivered through the MPU in the low Zn treatment. Moreover, mycorrhizal contribution to the shoot was comparatively constant, whereas the direct pathway uptake increased Zn uptake dramatically. The contribution of MPU decreased significantly with increasing soil Zn concentration. Emphasizing the relative contribution by MPU to the percentage of Zn absorbed in the 76R genotypes [4]. The mycorrhiza concentration of Zn was highest to a value of 21.7 μg with an application of 20 mg Kg-1 of Zn. The value of DPU increased with the contribution of MPU both in proportion and amount. Here in this study is to quantify the contribution by MPU to shoot Zn uptake in 76R genotype and to investigate whether the increasing soil Zn concentration leads to an increase in the Zn uptake through MPU. By using the same type of soil there is no effect of Zn fertilization on mycorrhizal colonization [4]. There are small growth depressions in the AM plants. This is possibly due to carbon drain by fungal colonization in the AM plants. But this result is not of big importance concerning the calculation for the MPU for Zn in the present study.
A large amount of Zn passes to the plant through MPU. Quantification of the amount of Zn uptake by the MPU pathway is not done before. Even though colonization by AM fungi, did not significantly increase uptake of Zn by fungi [4,11]. The MPU pathway of Zn uptake can be masked by plant tissue Zn content since the MPU and DPU are estimated separately. This is how while comparing Zn concentration in the mycorrhizal and nonmycorrhizal pathway uptake appeared inactive. Some factors like plant species, soil type, and chemistry, inoculum potential, size of the hyphal compartment, soil nutrients particularly P and Zn influence the uptake of Zn through MPU. Moreover, the methods of Zn determination and concentration and availability of Zn in soil, etc influence the MPU activity [4,11].
Irrespective of the soil Zn concentration there was a positive mycorrhizal P response in this study [4]. Beyond this, we cannot speculate the DPU and MPU as there is no distinct separation between these two pathways. It is observed that a larger proportion of P is transported to the plant than the added Zn [4]. Though a relative contribution and amount of Zn taken up via DPU and MPU are studied. Besides the interactive study of P and Z uptake without separation of pathways of uptake and genotype and other plant species. Thus, it is concluded that the interplay between DPU and MPU of Zn and P is highly complex and due attention has to be paid for further research. Increased in the root and shoot Zn uptake increased the Zn addition on both rmc and 76R genotypes of tomato. The percentage of shoot Zn concentration through the MPU pathway decreased at high soil Zn. The amount of Zn taken up by the MPU did not increase. The increase in total uptake is by the DPU. At low soil Zn supply, Zn is a limiting nutrient for uptake by the plant and the MPU has a significant role in Zn uptake. Negative MPU Zn responses are suggested when AM colonization of Zn present in the soil at toxic levels i.e., tissue Zn is less in AM plants than in the non-mycorrhizal ones. Watts-Williams et al. 2014, did not observe increased biomass in the AMF genotype at high Zn. But at high Zn, the relative proportion of Zn delivered by the MPU and transferred to the shoots of the AM genotype reduced significantly.
MPU is active when Zn supply is low. An important repercussion for crop growing in Zn deficient soil. The relative contribution by the MPU was reduced in huge amounts while the activity of DPU increased as the increase in the soil Zn supply. Furthermore, a comparative study between mycorrhizal and non-mycorrhizal plants cannot tell us about the activity and interplay between MPU and DPU. Independent study is required for this. The DPU in bread wheat is significantly affected by Zn level. The amount of Zn delivered did not vary between Medium and High Zn application but significantly lower at low Zn application. In increasing, Zn application increased straw and plant content of Zn. At high Zn, the amount of Zn delivered by DPU is 46 and 9 times higher than at low Zn respectively with straw and total plant uptake in Barley. A decrease of the MPU from 12.3 to 7.2% at low and medium Zn to high plant is observed. A 33-to-20-fold higher DPU in straw and the total plant was observed at high Zn to low Zn respectively. The increment in the MPU of grain is increased by 360% from low to high/medium Zn. The MPU to straw and total plant progressively increased from low to high Zn. Zn application significantly affected the DPU of Zn with grain, straw, and total plant uptake. The amount of Zn delivered by DPU to the grain significantly increased between low Zn and high Zn. The DPU to straw progressively increased with increasing Zn application. At high Zn, the DPU to straw and the total plant was 46 and 9-fold higher than at low Zn respectively [2].
About 24.3% and 12.7% of the above-ground Zn uptake respectively in wheat and Barley is contributed by MPU of Zn. In the lowest Zn addition, the highest uptake is observed in barley and the wheat with the highest supply of Zn. Increment in the grain yield of bread wheat is increased by AMF [2]. Plant receive Zn in the form of free ions such as (Zn2+ and ZnOH+). Numerous factors limit the Phyto availability of Zn like total Zn concentration, high organic matter, high CaCO3, neutral or alkaline pH, low redox conditions, high micronutrient or macronutrient, high concentration of ligands binding organs Zn complexes [2]. Zn can be also toxic to plants when applied in excess amounts [12]. Increasing acquisition of Zn in soil deficient in Zn has been studied [10,13]. Release of new crop varieties has been bred in Southeast Asia that can accumulate higher Zn concentration both in the straw and grain [14]. The mean value of the percentage of colonization in bread wheat and barley is 53 and 46 percentages, respectively. And this result showed the percentage of colonization significantly varied with Zn application. In bread wheat a decrease in mycorrhizal colonization by 16% with increasing soil Zn concentration from low/medium to high Zn. Contrastingly, barley root colonization by AMF is higher at low Zn and high Zn with a mean value of 51% than at medium Zn with a mean value of 36% [2]. AMF inoculation and Zn application have a differential response to yield, yield component, and biomass. In bread wheat, the above-ground biomass (grain+straw+chaff) was greater at medium Zn than at low and high levels. Moreover, the above-ground biomass did not vary with the application of AMF. The grain yield, number of kernels per spike, spike fertility index in bread wheat as affected by AMF inoculation. The grain yield was 21% higher in the inoculated plant (+M) than in the uninoculated plant (-M). Straw biomass and mean kernel weight were modified by Zn application with the value decreasing from 8 to 3% respectively from low/medium Zn to high Zn. In barley, none of the parameters was observed to be significant with an application of AMF and Zn [2].
There is a knowledge gap in the quantification of the contribution of the MPU in a wide range of soil Zn concentrations. It is also aimed to study the effects of AMF inoculation on the economic yield and plant nutrition of different plants. It is discovered that MPU contributed 24.3 and 12.7% of the soil Zn concentration. In bread wheat, negative colonization was observed with increasing soil Zn concentration. But the response is variable in Barley. Research conducted by Watts-Williams and their groups showed that decreasing mycorrhizal colonization with increasing Zn in soil [15]. Similarly, yield increment up to 18% is observed in several bread wheat genotypes when inoculated with R. irregular is (Pellegrino et al. 2015).
A large number of total Zn can be taken up by the MPU. It is discovered that a quarter of the Zn in bread wheat and one-eighth of Zn in Barley is contributed by MPU. The present findings suggested that MPU uptake of Zn is important to cereal Zn nutrition in a range of plant species including the plants earlier mentioned. By contrast, the data tracing from the MPU of Zn in tomato Zn contributions remains constant across the wide range of soil Zn concentrations. In the previous study, AMF are unable to regulate the amount of Zn via MPU even when the Zn is in excess [4].
Improvement in the Zn nutrition is one of the benefits of using mycorrhiza that improved the Zn concentration in edible parts of the plant including the grain [16]. In bread wheat, the reduction in the grain Zn concentration is compensated by increased grain yield in the mycorrhizal plant. Which leads to total Zn uptake irrespective of mycorrhizal plants. However, the increased grain Zn concentration is the important factor of increased biofortification outcome. Although there is a high amount of application of Zn for example 17 mg Zn Kg-1 soil in Medicago truncatula, 25 mg Kg-1 soil in tomato, and 50 mg Kg-1 soil in red clover no significant effect is observed in biomass and yield [17]. A high level of Zn is found to be a protective effect in the plant which depends upon the cation exchange capacity of the soil [18]. Although there are differences in MPU between wheat and barley at high Zn concentrations it is worth some to note that the contribution of MPU is similar (70 and 66 μg Zn in bread wheat and barley, respectively). With the change in the Zn availability in the soil, there is a difference in the partitioning of the MPU. In wheat and barley, 86% and 44% of the Zn are allocated to grain respectively [15].
The Role of Indigenous Mycorrhiza in Food Security
Plant production with above-ground or below-ground interactions helps to address food security. Two main strategies like increasing production by minimizing the gap between potential and actual production and reducing yield losses due to pests and disease attacks are practiced [19]. Mycorrhiza actively participates in the below-ground interaction. There is a huge scope of improving below-above ground interaction to enhance food security. Godfray et al. (2010) [20] summarize four main issues of above and below ground interaction like increasing production limits, changing diets, closing the yield gap, and reducing wastes. AMF could improve food security by increasing agricultural production through improved phosphate acquisition and improvement in disease or drought resistance [21]. The world population is reaching 9 billion by 2050. The application of mycorrhiza has a role in achieving the goal of food security. But two criteria effectiveness and safety rules the application of mycorrhiza in soil. The establishment of the mycorrhiza in soil and their pre-adaptation steps affect global food security [21]. Colonization with AMF is common in most of the plant in the field. Application of exotic AMF is presumed to be effective when the effect of indigenous performance of AMF is low. Nonetheless, there are no established criteria in measuring the performance of indigenous AMF in promoting plant growth. For each soil there is a unique mycorrhizal inoculant not affected by the colonization level of indigenous AMF or soil P. There is no compensation in the indigenous mycorrhiza with an application of exotic strain, however, the role of indigenous mycorrhiza was never negative. But both positive and negative roles were recorded in the inoculation of the exotic strain of mycorrhiza. Soil management factors limit the performance of the indigenous mycorrhiza which is harmless to plant [22]. Host specificity of AMF is observed with infection by multiple species (Van Tuinen et al. 1998; Opik et al. 2009).
The increase in global food demand is increased by the use of indigenous mycorrhiza. In a treatment applied with mycorrhiza increased P concentration in shoot than non-mycorrhized condition [23]. Application of AMF in field crops acts as biofertilizers and farmers decrease the number of phosphatic fertilizers to the crop field [24]. For instance, a substantial amount of P is reduced in Welsh onions and leads to achieving marketable yield under field conditions with the application of mycorrhiza. It is still debatable whether AMF application increases the direct transport of P to the plants or not [25]. The incorporation of indigenous AMF in the soil increased AMF propagules and increase the yield [26,27]. Based on the previous year’s experiment the use of mycorrhiza in the current year application of P can be reduced to half [28]. However, it is observed that not all the mycorrhizal experiments showed positive results it could be neutral or negative too [29]. So, the nature of such mycorrhizal experiment is affected by the type of AMF, plant type, growth stage, growth condition, soil biotic and abiotic properties [30-33]. Still, it is a matter of debate whether an application of AMF is effective or not [34-36]. The soil in an area contains a huge number of AMF propagules [37,38] and helps in global food security. The advantage of exploiting the indigenous source of AMF is adapted to local environments and able to promote the plant than exotic species [39,40]. There are few examples of increased crop yield after AMF are inoculated in the field [21]. Different soil management practices like fertilization, soil sterilization, excessive tillage, etc harm the performance of indigenous AMF [41,42]. So, a study in the investigation of the low performance of indigenous AMF and exotic strain could be organized [43]. To the best of our knowledge, there is no method for studying the performance of the indigenous AMF. Amendment with AMF is possible by mutualistic interaction with fungal species and plant roots. This relationship is looking forward to the greatest challenge of society which is food insecurity.
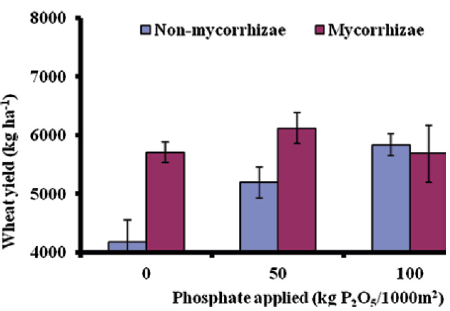
To manage the indigenous AM fungal spore soil and crop management factors is important in association with carbon amendment for soil. Food insecurity is one of the biggest challenges [44]. The yield of wheat in mycorrhizal and nonmycorrhizal is shown in Figure 2. Where three different levels of phosphate application have different responses to yield. At zero P application, the mycorrhizal wheat plants are far better than the non-mycorrhizal plant. A similar response is observed when mycorrhizal plants and non-mycorrhizal plants were treated with phosphate @50 Kg P2O5. However, the response was not significant enough when the application of phosphate @100 Kg P2O5[44]. This result signifies that mycorrhiza exhibits a beneficial response to soil with low P and increasing the concentration of P2O5 in the soil does not show a significant response.
Figure 2: Wheat yield in mycorrhizal and non-mycorrhizal treated treatments under different P2O5 levels [44].
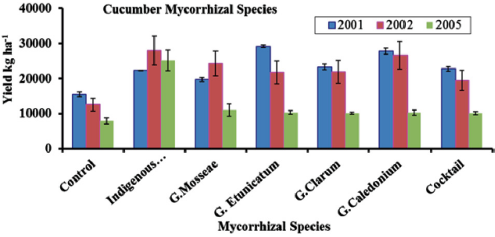
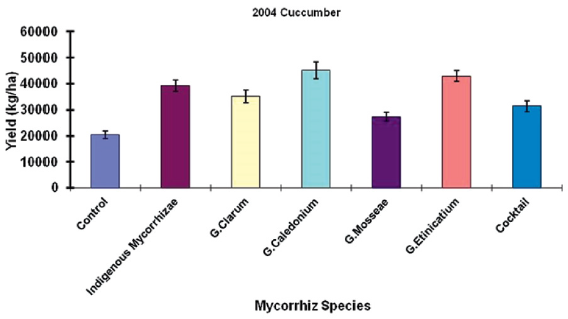
A study conducted by Ortas et al. [44] presented the response of cucumber against a different strain of mycorrhiza including with indigenous were tested. This experiment was conducted for three years, and the indigenous mycorrhiza has equal effect as other types tested in the years 2001 and 2002. While in the year 2005, the efficacy of using indigenous mycorrhiza was higher than the other five different strains used in this study. So, the use of indigenous mycorrhiza is widely applicable in a variety of soil types (Figure 3). In Figure 4 results have been shown about the response of cucumber with five different strains of mycorrhizae, indigenous mycorrhizae, and control. Indigenous mycorrhizae, G. caledonium, G. etinicatium has a similar response and significantly different from other strain of mychorrhizae. So, host specificity of mycorrhiza is very important to explore [44].
A symbiotic association is formed between the roots of the majority of plants and mycorrhiza [45]. This relationship provides water, nutrients like P, N, and other salts and metals including Zn to the plant, and in return, the plant provides carbohydrates to arbuscular mycorrhizal fungi (AMF). There is variation in AMF due to species diversity, season, biogeographical history, and environmental conditions. Plant in the family Amaranthaceae, Cruciferae, Caryophyllaceae, Chenopodiaceae, Juncaceae, Polygonaceae, and Cyperaceae do not form any associations with mycorrhiza [46]. The symbiotic association between fungi and plants plays a vital role in the uptake of P. Many studies have confirmed that mycorrhizal symbiosis can change the symbiosis of its host plants. Agricultural production has been declining due to faulty agricultural practices. In this context, a practice of integrated nutrient management (INM) has been adapted. This INM includes the inclusion of organic and inorganic fertilizer with mycorrhiza as a biofertilizer [6].
Interaction Between Zinc and Phosphorous in Presence of AMF
In bread wheat, the concentration of Zn both in the grain and straw is affected by the interaction between AMF and Zn. In the grain yield, non-mycorrhizal plants showed higher Zn concentration than mycorrhizal plants. Grain Zn concentration ranged from 28 to 101.18 μg Zn g−1. In straw, the low Zn and the high Zn concentrations varied from 7.8 to 256.3 μg Zn g−1. At high Zn concentrations were higher in the non-mycorrhizal plants than the mycorrhizal plants. In barley grain and straw were also unaffected by the Zn application. Similarly, for the straw component, the low and high value of Zn concentrations were ranged from 11.73 to 472.6 μg Zn g−1. While the range for grain Zn concentration is closer than straw component 19.14 to 114.16 μg Zn g−1. The Zn content of bread wheat is affected by the interaction between AMF and Zn. While Zn content in the straw was affected by the AMF and Zn application. Irrespective of the AMF inoculation the grain Zn content was increased by 1.5 times in Barley. On average the grain Zn content varied from 24 to 60.8 μg plant 1. The accumulation of Zn in the non-mycorrhizal and mycorrhizal plants was respectively 77.7 and 110 μg plant−1. An observance of lower accumulation of Zn is observed in mycorrhizal plants than non-mycorrhizal plants. A similar trend was observed in straw Zn content. In barley grain and straw, Zn is increased by Zn application. It is reported that Zn content in the grain increased by 280 and 69 percentages. The content in Barley grain Zn concentration at low, medium, and high Zn is 12.2, 46.4, and 78.4 μg plant−1 respectively. A similar observation was recorded in straw Zn concentration [2].
The interaction between AMF inoculation and Zn application affected P content in grain and straw. The P content of the straw is significantly increased with AMF inoculation. The increase in P uptake is 28% in mycorrhizal and 25% in non-mycorrhizal plants. AMF application changes the concentration of P at low and medium Zn but not in the high Zn. The mycorrhizal plant grown at lower Zn availabilities has the P content was similar [2]. In the nonmycorrhizal plants, the P content in straw decreased from 2.9 to 2.1 respectively at the low, medium, and high Zn respectively. In straw inoculation by AMF decreased in all Zn applications. But the rate of decrease is varied to Zn application. Zn applications ranged between 44 to 29% at low to high Zn applications, respectively. A similar pattern was observed in straw Zn content with values in the low, medium, and high Zn respectively 22.5, 287.9, and 977.8 μg plant 1. Interaction between AMF and Zn application affect P content in the grain did not vary according to the Zn application whereas P content in straw is affected with the application of Zn and AMF. In detail P content in the straw is found to be increased by 26% in a non-mycorrhizal plant than in the mycorrhizal counterpart [2].
The involvement of soil properties and plant nutrition is the major input of microorganism-driven sustainable agriculture [6]. The major function of beneficial microorganisms is the production of plant growth regulators, protection against root pathogens, nutrient uptake acquisition, increased availability of nutrients, etc. According to their relationship with microorganism are divided into several categories. For instance, parasitic, saprophytic, symbionts, and mutualistic symbionts. The mutualistic symbionts are also known as biofertilizers because it brings the function for the plant that they cannot perform themselves. The host plant receives mineral nutrients from the microorganism and the microorganism receives the photosynthetically derived carbon compounds [47]. Among all these microorganisms vesicular-arbuscular mycorrhiza (VAM) is a beneficial fungus that plays an important role in soil nutrient dynamics and improving soil physical, chemical, and biological properties [48]. Recently throughout the world, the mycorrhiza association is a widely abundant symbiotic relationship [49]. The proper functioning of the mycorrhiza needs soil, host plant, AMF, and environmental condition suited to the growth and development of the mycorrhiza. Inhibition of one of the components inhibits the functioning of the host plant interaction [6]. A series of root fungus interactions and colonization to the host exists. The hyphal network initiated the mycorrhizal colonization process that produces soil-borne propagules which are also called large resting spores. An appressorium is usually formed on the epidermal cell which passed through intracellular spaces and then enters root tissues through cortical root layers. When the Hyphae reach the cortex, they grow into cells as dichotomous branching which forms a tree-like structure called arbuscules [50]. Arbuscles formation mimics a large surface of cellular contact between both symbionts. The age of the arbuscles is varied from 4-14 days. Low to medium mobile nutrient elements are absorbed by the hyphae and bridged through the nutrient depletion zone to the bulk soil by the arbuscles [51].
The early record of the occurrence of AMF in rhizomatous tissue, scale-like leaves, and vascular systems is shown [52]. AMF colonization record is well documented as most of the research work on AMF has been focused on the interaction between AMF and plant roots. Potato seeds were inoculated with AMF fungi. In the present work, a hypothesis is raised in the formulation of whether the AMF fungi influence underground seed tuber. As these tuber propagules comprise the principal material for the propagation and underground seed tuber. In the presence or absence of AMF. Confirmation of the mycorrhizal colonization is achieved with the presence of hyphae, arbuscles, or vesicles [53]. The accumulation of dry matter varied in the interval of the growth 40, 60, and 80 days. Similarly, there is differentiation in the accumulation of dry matter between 20 and 40 days and reached a maximum between 60 and 80 days of growth respectively in the AMF colonized to non-colonized [53]. In this paper, an attempt has been made about mycorrhizal symbiosis in plant roots by colonization, soil P dynamics in the rhizosphere, and mycorrhizal mechanism and pathways involved in P availability and uptake [6].
Phosphorous (P)
The plant requires an adequate amount of P to enhance shoot and root growth and eventually promote early maturity which in turn helps to increase water use efficiency (WUE) and the amount of crop yield. It is the significant element that helps in the store and transfers energy produce by photosynthesis [54]. Although P is the second macronutrient required in relatively large amounts by plant next to N, the plant has difficulties to uptake because of low solubility, mobility, and fixation in the soil [55]. The presence of Fe and Al fix P in the soil. Highly weathered utltisol, alfisol, and oxisol and Ca phosphates fix P in calcareous soils. Moreover, rooting type, soil properties, and soil moisture availability, etc influenced the availability of P. As the availability of P is governed by many factors diffusion process is the major which is dependent rather than mass flow and interception.
Phosphate is one of the key growth elements for all plants. Plant derive soil phosphorous through DPU and MPU from the rhizosphere. Rapid immobilization of cations often limits the phosphate in the majority of soils. Phosphorous supply is limited caused by the formation of depletion zone around the plant root through DPU. In developed countries, a large amount of P is added to the agricultural soil. Which ultimately causes environmental pollution and hinders the uptake of other nutrient elements such as Zn, Cu, Manganese (Mn). The hyphal network surpasses the depletion zone and accessible to a greater area of soil P uptake. With mycorrhizal symbiosis promote the use and uptake of P [45]. Although P is the second most important nutrient element for plant growth and development, it is one of the most difficult nutrient elements to uptake due to low mobility, low solubility, and fixation in soil [6].
Relationship between Agronomic Traits and Pathogen Defense System with AMF
Plant root secretes hormones strigolactone to stimulate colonization, spore germination, hyphal branching, and metabolism [56,57]. Similarly, the release of mycorrhizal factors also known as lipochitooligosaccharide signaling molecules is recognized by host plant receptors [58]. A tradeoff between agronomic traits like plant growth and nutrition response and symbiosis as well as there is a tradeoff between AM symbiosis and disease resistance exist [45]. By AMF colonization in rice receives more than 70% of the phosphate via the MPU [59]. Growth response is positive due to increased phosphate uptake through the MPU, which can also result due to other growth-limiting nutrients like nitrogen [60] (Smith and Read, 1997). A positive effect on Nitrogen fixation by legume AMF symbiosis is observed than non-AMF counterparts [61,62]. Plant water relations [63,64], improved phosphate nutrition [65] and abiotic stress like drought and salt stress tolerance are some of the benefits of using AMF in a crop. Accumulation of sugars in the root is one of the mechanisms of overcoming salt stress in the plants [66], enhanced nutrient acquisition [67,68], and maintenance of K+:N+ in roots [69]. Similarly, AMF is helpful to reduce the negative effect of heavy metals like Pb, As. Glutahione-S- transferase which transforms the toxic form of as into a non-toxic one. In high P application AMF alleviates symptoms of phosphate toxicity [70]. The transport of sulfur-containing compounds via MPU minimizes sulfur starvation in plants (Allen et al. (2009); Sieh et al. (2013). The application of AMF to crop plants reduces many diseases [45]. The competition between root pathogen and AMF in the rhizosphere for food and space is the major reason for the reduction of the pathogen in the root [71]. It has been observed that the induction of systemic acquired resistance is playing a major role in the plant affected with AMF [72].
The Exchange Between AMF and Plant Disease Resistance Traits
In disease resistance, AMF has a positive effect, for example, SAR-like defense response [73] the growth response based on genotype-dependent phenotype is observed [74]. So far very little is figured out about the induction of disease resistance by genotypic variation in AMF [75]. Even though the mechanism of AM-mediated disease resistance due to genotypic variation is not fully understood. The exploration of better disease management strategies in the rhizosphere highlighted the importance of the AMF symbiosis [45]. It has been suggested that AMF induced immunity is beneficial. Thus, a gap between crop breeding programs and symbiosis needs to be addressed [45]. The MPU generally increases the phosphate uptake in phosphate-limited conditions [76,77]. But AMF always does not have positive effects sometimes there are growth depressions [78]. Early growth depressions are very detrimental to plant. But in the case of AMF symbiosis, they fulfill the reproductive goal, so it is enough time to record this.
The key drivers of the AM symbiosis in the ratio of carbon provision to phosphate acquisition. The efficiency of this relationship is viewed as the involvement of MPU or DPU. Generally, DPU is not affected by mycorrhizal colonization. The added phosphate to the rhizosphere is through extra phosphate. The MPU pathway is helpful to compensate for the phosphate uptake depending upon the amount and functioning of the DPU. Moreover, the amount of phosphate uptake by DPU and MPU is additive. A result of the reduction of phosphate uptake is possible depending on the available phosphate and genotype and AMF. Mycorrhizal pathway uptake can fail to reduce phosphate uptake [79]. In some studies, the phosphate transporter gene in the DPU was shown to be downregulated. It is argued that the AMF species do not provide enough phosphate in exchange for the host plant carbon. Such types of depression are not due to the lack of phosphate, but MPU and DPU are partially exclusive. So, utilization of AM symbiosis in sustainable agriculture necessitates making the DPU and MPU additive than partially complementary (Smith et al. 2001).
There is a trade-off between plant roots and AMF symbiosis. The similarity between the infection of biotrophic pathogen and AMF colonization is very common. Biotrophic pathogen penetrate host cell through an intracellular infection structure called haustoria. Also, AMF have a similar structure called arbuscules [80]. The study of transcriptome showed similar pathogen haustoria and AM arbuscules [81]. The similarity between the levels of gene expression reflects the similarities between haustoria and arbuscules. It can be presumed that the co-evolution of these two structures happened before the onset of the disease.
Phosphorous Induced Zinc Deficiency
A high dose of phosphorous (P) application caused to induces a lack of zinc (Zn) in crops grown under Zn-poor soils. A study is conducted with P- induced Zn deficiency in durum wheat (Triticum durum L. ‘Carpio’) showed that Zn deficiency in 31 calcareous soils and 16 non-calcareous soils with varying levels of soil P [82]. In calcareous soil, P-induced Zn deficiency is less pronounced than non-calcareous soil because the calcite absorbed phosphate. In control plants, grain zinc concentration is negatively correlated both in the calcareous and non-calcareous soil. But this study shows that the correlation is stronger for non-calcareous soil. So co-application of Zn and P induced maximum yield and Zn bioavailability at low application [82]. This type of interaction mainly depends upon mycorrhiza in contact with the surface of the root. The solubility or the bioavailability of both nutrients is highly influenced by the P- and Zn-reactive soil [82]. There is still a rumor about the enhancement of Zn absorption by P application or not. Similarly, the negative interaction between P on Zn uptake in wheat grown on artificial media increased with increasing the ratio between Fe oxide and Calcite [83]. Under the rice (Oryza sativa L.) wheat (Triticum aestivum L.) system the use of higher P and Zn levels increased in rice grain and rice straw. Plant genetic and crop management factors both influenced the Zn uptake under the rice-wheat farming system (Amanullah et al. 2020). In a study, a hypothesis is raised whether the response of native and soilapplied P and Zn and its severity depends on soil properties or not.
Continuous uptake of Zn from the soil caused a deficiency in wheat and rice grain and straw content of Zn. Also, there is decreasing the residual concentration of Zn amount in the soil due to continuous cropping of rice and wheat hybrid varieties (Amanullah et al., 2020). Integrated application of Zn and P @ 10 and 15 Kg/ha Zn and @ 80 and 120 Kg/ha P is recommended respectively. Also, the response of Zn biofortification depends on the rice varieties. For example, coarse rice varieties are more responsive to Zn biofortification than fine rice varieties [84]. The plant absorbs Zn as a cation (Zn2+) and P in the form of anions of H2PO4 -1 or HPO4 -2 [84]. There are attractions between these two cations and anions to form chemical bonds both in the soil and plant. In case P binds a large amount of Zn which is normally available to plant results in the phenomena so-called as P induced Zn deficiency. This happens reduction in shoot Zn concentration and reduced plant growth [85]. Coarse rice varieties extract more Zn from the soil than fine rice varieties. It is recommended to supply an additional amount of Zn in the wheat crop grown in a sequence with coarse varieties of rice [85]. The soil types conducive to Zn deficiency are calcareous, heavy clay, sandy, and alluvial soil. Soil that has low organic matter and high pH possesses Zn deficiency problems. Reduction in Zn availability pertains to soil with waterlogging and the soil were restricted root growth. In soil, some phenomena such as cool wet weather, high soil nitrogen, P and Cu, and low light intensity caused Zn deficiency. Such type of soil deficient in Zn shows reduced grain yield and quality and leads to human Zn deficiency mainly in the countries based upon cereal-based diets. This can be a major reason for declining human health [85]. The concentration and availability of the Zn in plants are affected by P, nature, and properties of soil, environmental factors, and water availability. Very little research is going on in the integrated use of Zn and P interaction and Zn biofortification in grain and straw. Therefore, this study is designed to show the effect of Zn and P on Zn biofortification in plants, humans, and their interface.
The maximum (20.04 mg Kg-1) and minimum (16.32 mg Kg- 1) grain Zn concentration in rice is observed with the application of phosphorous @120 Kg ha-1 and P control plots respectively. Similarly, the maximum value of straw Zn concentration (21.89 mg Kg-1) is recorded in treatment with P application @120 Kg ha-1. Likewise in straw, the minimum Zn concentration in rice is (19.72 mg Kg-1) with the application of P @40 Kg ha-1. With increasing P levels from 80 to 120 Kg, ha-1 increased grain Zinc concentration in both grains and straw [84]. In contradiction to the application of the above result of P resulted in the decline in Zn in both the shoots and roots. Such type discrepancies are probably due to the different genotypes, soil, and environmental factors [86]. Co application of foliar-applied P and Zn @3% and 0.3% respectively improved the growth, yield, and yield attributing parameters in maize. Application of P and Zn at the boot stage improves growth and increases profitability and productivity under moisture stress in semiarid climates [85]. The soil-applied P has a low diffusion coefficient. So, soil-applied P is very low, and a plant cannot get P when needed. Therefore, foliar application and P retention through stomata are important. Foliar application of KH2PO4 delayed leaf senescence and increase winter wheat grain yields during hot dry summers [85].
An excess level of P imposed Zn deficiency in several plants [85]. One of the widely studied interactions in the plant is the interaction between P and Zn. There are two possible fates of these interactions whether it leads to increment or decrease in the Zn concentration in the plant. An increase in the application of P is likely to reduce Zn concentration in grains or plants. A higher application of P and a lower amount of Zn increases P toxicity in plants showing symptoms similar to Zn deficiency. The increment in P supply caused impairment in the Zn translocation from roots to the upper parts of the plants. In-plant root due to an excessive amount of P caused Zn to bind with root cells and then unavailable to plant. In another case, a high concentration of Zn in root cells results in the unequal distribution of Zn between roots and upper parts of the plant [87].
Two essential elements which affect crop growth and development are P and Zn. But in several cases, these nutrient elements act as antagonistic mutually [88]. This leads to a further reduction in yield, nutrient uptake in several crops due to incompatibility between P and Zn [88]. Independent responses of a nutrient element are reported under the influence of arbuscular mycorrhizal fungi (AMF). So, application of AMF not only promotes the nutritional status of the plant but also promotes the independent functioning of plant nutrients. The benefit of using AMF is more when plants were grown under P/Zn deficiency. Some soils which are high in P can affect the uptake of zinc in the plant. So, AMF colonization is expected to improve the availability of micronutrients [88]. Paying attention to this aspect a depth study is undertaken in AMF, P, Zn, and their interaction. Mainly there are two types of interaction that persist in the soil-derived Zn and P, viz: antagonistic and synergistic interaction [88].
In between two nutrient elements, P and Zn the presence of one limit the availability of the other. Generally, there is the presence of a high amount of P in soil due to soil application [88]. Which suppresses the availability of Zn in soil? In P and Zn interaction most of the interactions occur as antagonism. It is still controversial whether the interaction occurs above or below ground. Halder and Mandal (1981) [86] reported that application of P induced Zn deficiency in both shoots and roots. The lower amount of Zn in the shoot is not because of the low transportation of the Zn from vascular tissues. A decrease in the concentration of Zn, copper (Cu), manganese (Mn) in leaves of the soybean is observed in the solution culture at high pH. But at low pH, a reverse condition is observed. Precipitation of the Zn phosphates in the root may be one of the reasons for antagonism. Interaction between Zn and P occurs in the plant. An increment in the Zn concentration in the roots and a decrease in the Zn concentration in the shoot are the consequence of a high level of P supply. This signifies that the interaction occurs within the root due to the sidelong rupture of vascular tissues. Not only in the limiting Zn but also observe the decrease in the concentration of Copper (Cu), Iron (Fe), and Manganese (Mn) due to high P both in the roots and shoots. A high level of one nutrient hinders the uptake of others in marginal quantity. The Zn-induced deficiency of P is a very rare phenomenon because hardly there are any pieces of evidence of artificial Zn application in plans’ shoot or root. When the application of VAM in roots and shoots increased P uptake and plant growth markedly.
Acquisition of several plant nutrients like N, P, K, Mg, Cu, Ca, and Fe, improvement in the soil quality, and tolerance to abiotic stress such as drought, heat and increased plant resistance to many biotic and abiotic stress factor is possible by application of AMF [88]. There are two types of interaction one is synergistic, and the other is antagonistic. In synergistic interaction, there is a positive effect of one nutrient element to another and in antagonistic, there is a negative influence of one nutrient element to another one. In positive interaction, there is an increase in crop growth and productivity with the help of associated elements. In negative interaction, there is a decrease in the growth, development, and yield of the crop with the application of other nutrients. The most important antagonism occurs in Zn by excess application of P. Increasing P supply caused an increase in root Zn amount and decrease in the shoot. Which advises that Zn and P interaction occurs in the root. There is a possibility of the rupture of sidelong Zn transport from vascular tissue from root to the upper part. The movement of Zn within roots and roots to shoots is checked by the formation of sparingly soluble Zinc Phosphates [88]. There could be a possibility of formation of P/Zn complex in roots which prevents the movement of P to the shoots in high Zn supply. Independent actions are needed to absorb P or Zn nutrition by the host plant [88]. In mycorrhiza, plants have greater tolerance to the deficiency of P and Zn [89]. The three different genes MtPT1, MtPT2, and MtPT3 are involved in the direct pathway uptake of P. Expression of genes encoding phosphate transporters (PTs) are highly expressed when plants are grown under low soil P conditions [89]. By the development of symbiosis between roots and AMF caused downregulation of PTs. Loss of one of the PTs gene MtPT4 leads to impairment in the mycorrhizal symbiosis. This phenomenon is happening because of arbuscular death. With the application of 5, 20, and 50 mg P Kg-1 increase the shoot dry weight, root dry weight, shoot P concentration, and P contents in application with 0.3, 4, 5.8, and 15 mg Zn Kg-1 [89]. The gene MtZIP5 expression is induced by both mycorrhizal colonization and soil Zn availability. Contrastingly, the expression of MtZIP2 is up regulated in non-mycorrhizal roots and increased with soil Zn availability. AMF has a protective role in examining shoot biomass and Zn concentration.
The colonization of AMF is observed in the control treatment. But the AMF activity decreased substantially with increasing soil concentration of P by 8.7%. Similarly, the expression of the α-tubulin gene in R. irregular is decreased with increasing soil P application. So, the mycorrhizal colonization is decreased in any of the mock-inoculated plants [89]. The expression of the gene MtZIP2 is affected by the P and Zn applications. Similarly, the expression of genes MtZIP5 and MtZIP6 is governed by the interaction between Zn and P. The direct pathway phosphate transport (PT) gene MtPT1 was downregulated in the mycorrhizal plants. The expression of the two genes is decreased by the addition of the P doses. Symbiosis has a major benefit of forming mycorrhizal associations on the growth and development of the plant. A study about symbiosis is done in various areas. The application of P 20 to 30 mg Kg-1 showed a positive response to mycorrhizal association. The biomass and MP are active in flax (Linum usitatissimum) and transported a substantial amount of P to the plant. This is how the response of AMF can be positive, neutral, or negative cannot be used to estimate the P uptake.
According to Nguyen et al. (2019) showed decreased mycorrhizal colonization in the root with response to added P in the soil. About soil Zn mycorrhizal colonization increase with MPU activity. There is an increase in Medicago biomass both at deficiency and toxic concentration of Zn applied. This is due to the dual roles of AMF under low and high levels of Zn in plants. Under low concentration of Zn mycorrhizal plant increase the Zn concentration and at a high level of Zn blocked the transport to the shoot. The results as shown by Nguyen et al. (2019) [89] confirm that mycorrhizal colonization benefits the Medicago plant at low P concentration. Besides shoot P contents in mycorrhizal plants are maintained across the wide range of Zn availabilities at high soil P treatments. By contrast, the shoot P content was negatively influenced. Mycorrhizal colonization does not have a response once the Zn reached a very high concentration in plant shoot for example 40 mg Kg-1. At this concentration, mycorrhizal colonization cannot maintain plant shoot P contents. Such types of buffering capabilities of the mycorrhizal plant at varying level of Zn concentration is noteworthy [89].
Interaction Between Phosphorous and Mycorrhiza
Interaction between mycorrhiza, P, and host plant plays an important role in the uptake of P. Due to lower mobility of P in the soil there is a creation of the depleted zone. Further P in the soil has to be utilized quickly. The plant needs to bypass the depleted zone increasing further root activity. The distant transportation of P uptake in the mycorrhizal plants is mainly due to the absorption and translocation of P from distant areas. Generally, fungal hyphae absorb P in the form of orthophosphate and are conveyed as polyphosphate [90]. Increased absorption of P by MPU has been attributed to an increase in the surface area for absorption [91]. Hyphal fitness extended the advantage of increasing root surface area for greater absorption of the nutrient. An increment in the area of exploration by enabling the entry of hyphae into soil pores, which cannot be penetrated by root hairs. With the function of extra radical hyphae, there is rapid absorption of plant soluble P which caused a shift in the equilibrium. In comparison to non-mycorrhizal plants, mycorrhizal plants absorbed a much higher amount of P. This suggests that a higher affinity of a mycorrhizal fungus for phosphate ions at a lower threshold amount than do by the plant roots [92].
The negative potential created by H2PO4 caused the cell membrane to have negative electric potential. Generally, plant uptake P as ions which increase negative potential. So, some additional amount of energy is needed for the Pi uptake and requires high-affinity transporter proteins. So, the pathway is a high-affinity transporter which is more effective in the root apex. Loss of root hairs caused a reduction in the transporter protein activity and caused the decline of DPU. In another hand, there will be a creation of a depletion zone because of P uptake as orthophosphate (Figure 1). This leads to lower Pi concentration in the root rhizosphere and a zone of depletion is created [5]. MPU is an alternative strategy developed by a plant to uptake the P. MPU pathway brings soil from large volume and transported it to cortical cells evading the DPU [93]. The relationship between phosphate and mycorrhiza is ambiguous. A site with a large amount of extractable P may have a level of infection and many spores. Whereas a site with a low amount of P may have a low number of spores and colonization [6,94]. In contrast inoculation with VAM in the absence of added P increased available soil P because of the release of organic exudates in the rhizosphere. It is suggested that the mycorrhizal inoculum substitute soil P level in the plant equivalent to 30 Kg per ha [6,94].
However, in some cases there is a negative correlation has been found between the P and VAM fungi [95]. The addition of P fertilizers does not affect or decrease the level of mycorrhizal infection in a range of crops. This relationship is possibly due to the correlation with phosphate and the lengths of root colonized. High use of P alters root colonization especially reducing arbuscule for development and decrease mycorrhizal fungal biomass per plant. Plant-derived signals and formation of appressorium are observed in Pisum sativum at high P [96]. In a P surplus condition, a direct but possible less costly uptake pathway is preferred and the low colonization [97]. But production of secondary metabolites also known as strigolactones which mediate signaling for root colonization. Root colonization has a strong negative effect with high P supply in various species. At a low rate of application, the P does not have any significant effect. The effect of the P source is evidently from the rate is high. The high root length is always associated with the rock phosphate rock source which is expected to differ from the superphosphate. Thus, there is a difference in infectivity associated with the higher rate of superphosphate application could also have been due to the differences in rates of dissolution of superphosphate and phosphate rock to provide phosphorous in the soil solution over a given period [97]. There is antagonistic interaction between soil P and Zn when any one of the nutrients is in excess caused depletion of another one. Improved P nutrition has a dilution effect on plant growth and development. There is an additive effect of P and Zn nutrition in rice. The Independent function of P and Zn is performed by the application of mycorrhiza in soil [98-107].
An important role in the P nutrition is played by inorganic P to organically bound P. In the presence of mycorrhiza help in the P acquisition in the rhizosphere. Similarly, the P activity of VAM colonized soil was higher irrespective of the stages of the observation. An increase, decrease, or stable supply of mycorrhiza help in plant water use efficiency. This characterizes the positive effects of mycorrhizal colonization in terms of the improved P nutrition and larger biomass of mycorrhizal plants. This is how the contribution by mycorrhizal colonization towards plant drought tolerance could be the cumulative impact of nutritional, physical, physiological, and cellular effects posed by VAM [43].
Conclusion and Way Forward
The world population is reaching nine billion by 2050. The growing billions require a substantial amount of food to feed. To meet the food requirement application of mycorrhiza as an important biofertilizer is suggested. So mycorrhizal pathway of uptake of essential plant nutrients is recommended. Between two pathways of uptake, MPU has several advantages over DPU. Moreover, farming areas around the world are experiencing a major problem of P-induced Zn deficiency. Therefore, the incorporation of indigenous mycorrhiza could partly solve the problem. But a complete solution to this problem is still unavailable. The use of both exotic and indigenous mycorrhiza in the production of various plant products is the future of modern agriculture.
Acknowledgments
We highly acknowledge this article to the reader and thankful for all the commercial mycorrhiza producers and native farmers.
Funding
This review is jointly funded by the College of Resources and Environment, Huanzhong Agricultural University (HZAU), Wuhan 430070, China and Agriculture Research Station, Nepal Agricultural Research Council, Pakhribas, Dhankuta, Nepal.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Schausberger P, Peneder S, Jurschik S, Hoffmann D (2012) Mycorrhiza changes plant volatiles to attract spider mite enemies. Functional Ecology 26: 441-449.
- Coccina A, Cavagnaro TR, Pellegrino E, Ercoli L, McLagughlin MJ, et al. (2019) The mycorrhizal pathway of zinc uptake contributes to zinc accumulation in barley and wheat grain. BMC Plant Biology 19: 133-146.
- Smith SE, Smith FA, Jakobsen I (2003) Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol 133(1): 16-20.
- Watts-Williams SJ, Turney TW, Patti AF, Cavagnaro TR (2014) Uptake of zinc and phosphorous by plants is affected by zinc fertilizer material and arbuscular mycorrhizas. Plant Soil 376: 165-175.
- Smith SE, Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol 62: 227-250.
- Lalitha M, Kumar KA, Dharumarajan S, Balakrishnan N, Srinivasan R, et al. (2017) Role of vesicular-arbuscular mycorrhizae in mobilization of soil phosphorus. In Agriculturally Important Microbes for Sustainable Agriculture (pp. 317-331).
- Piliarová M, Ondreičková K, Hudcovicová M, Mihálik D, Kraic J (2019) Arbuscular mycorrhizal fungi–their life and function in ecosystem. Agriculture 65(1): 3-15.
- Cakmak I (2002) Plant nutrition research: Priorities to meet human needs for food in sustainable ways. Plant Soil 247(1): 3-24.
- Bhantana P, Timlin D, Rana MS, Moussa MG, Zhihao D, et al. (2020) How to Cut Down the Gap Between the Zn Requirement and Supply of Food Chain and Crop Growth: A Critical Review. Int J Plant Anim. Environ Sci (1): 001-026.
- Cavagnaro TR, Dickson S, Smith FA (2010) Arbuscular mycorrhizas modify plant responses to soil zinc addition. Plant Soil 329(1-2): 307-313.
- Watts-Williams SJ, Patti AF, Cavagnaro TR (2013) Arbuscular mycorrhizas are beneficial under both deficient and toxic soil zinc conditions. Plant Soil 371(1-2): 299-312.
- Palmgren MG, Clemens S, Williams LE, Krämer U, Borg S, et al. (2015) Responses of wheat to arbuscular mycorrhizal fungi: a meta-analysis of field studies from 1975 to 2013. Soil Biol Biochem 84: 210-217.
- Cakmak I (2008) Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant soil 302(1): 1-17.
- Pfeiffer WH, McClafferty B (2007) HarvestPlus: breeding crops for better nutrition.Plant Sci 1: 464-473.
- Watts-Williams SJ, Cavagnaro TR (2018) Arbuscular mycorrhizal fungi increase grain zinc concentration and modify the expression of root ZIP transporter genes in a modern barley (Hordeum vulgare) cultivar. Plant Sci 274: 163-170.
- Ercoli L, Schüßler A, Arduini I, Pellegrino E (2017) Strong increase of durum wheat iron and zinc content by field-inoculation with arbuscular mycorrhizal fungi at different soil nitrogen availabilities. Plant Soil 419: 153-167.
- Chen BD, Li XL, Tao HQ, Christie P, Wong MH (2003) The role of arbuscular mycorrhiza in zinc uptake by red clover growing in a calcareous soil spiked with various quantities of Zinc. Chemosphere 50(6): 839-846.
- Warne MSJ, Heemsbergen D, Stevens D (2008) Modeling the toxicity of copper and zinc salts to wheat in 14 soils. Environ. Toxicol Chem 27: 786-792.
- Orrell P, Bennett AE (2013) How can we exploit above–belowground interactions to assist in addressing the challenges of food security? 4: 432.
- Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, et al. (2010) Food Security: The challenge of feeding 9 billion people. Science 327: 812-818.
- Rodriguez A, Sanders IR (2015) The role of community and population ecology in applying mycorrhizal fungi for improved food security. ISME J 9: 1053-1061.
- Teranishi T, Kobae Y (2020) Investigation of Indigenous Arbuscular Mycorrhizal Performance Using a Lotus japonicus Mycorrhizal Mutant. Plants 9(5): 658.
- Smith, SE, Read DJ (2008) Mycorrhizal Symbiosis; Academic Press: Cambridge, UK.
- Srivastava P, Saxena B, Giri B (2017) Arbuscular mycorrhizal fungi: green approach/technology for sustainable agriculture and environment. In Mycorrhiza – Nutrient Uptake, Biocontrol, Ecorestoration; Varma A, Prasad R, Tuteja N (Eds.); Springer International Publishing: Cham, Switzerland: pp. 355–386.
- Elliott AJ, Daniell, TJ, Cameron DD, Field KJ (2019) A commercial arbuscular mycorrhizal inoculum increases, root colonization across wheat cultivars but does not increase assimilation of mycorrhiza-acquired nutrients. Plants People Planet 2019
- Arihara J, Karasawa T (2000) Effects of previous crops on arbuscular mycorrhizal formation and growth of succeeding maize. Soil Sci. Plant Nutr 46: 43-51.
- Karasawa T, Takebe M (2012) Temporal or spatial arrangements of cover crops to promote arbuscular mycorrhizal colonization and P uptake of upland crops grown after nonmycorrhizal crops. Plant Soil 353: 355-366.
- Oka N, Karasawa T, Okazaki K, Takebe M (2010) Maintenance of soybean yield with reduced phosphorus application by previous cropping with mycorrhizal plants. Soil Sci Plant Nutr 56: 824-830.
- Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84(9): 2292-2301.
- Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, et al. (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13: 394-407.
- Walder F, Van der Heijden MG (2015) Regulation of resource exchange in the arbuscular mycorrhizal symbiosis. Nature Plants 1(11): 1-7.
- Powell JR, Rillig MC (2018) Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol 220: 1059-1075.
- Renaut S, Daoud R, Masse J, Vialle A, Hijri M (2020) Inoculation with Rhizophagus irregularis does not alter arbuscular mycorrhizal fungal community structure within the roots of corn, wheat, and soybean crops. Microorganisms 8: 83.
- Hart MM, Antunes PM, Chaudhary VB, Abbott LK (2018) Fungal inoculants in the field: Is the reward greater than the risk? Funct Ecol 32: 126-135.
- Ryan MH, Graham JH (2018) Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol 220: 1092-1107.
- Rillig MC, Aguilar-Trigueros CA, Camenzind T, Cavagnaro TR, Degrune F, et al. (2019) Why farmers should manage the arbuscular mycorrhizal symbiosis? New Phytol 222: 1171-1175.
- Lekberg Y, Koide RT (2014) Integrating physiological, community, and evolutionary perspectives on the arbuscular mycorrhizal symbiosis. Can J Bot 251: 241-251.
- Kobae Y (2019) Dynamic phosphate uptake in arbuscular mycorrhizal roots under field conditions. Front Environ Sci 6: 159.
- Schreiner RP (2007) Effects of native and non-native arbuscular mycorrhizal fungi on growth and nutrient uptake of ‘Pinot noir’ (Vitis vinifera) in two soils with contrasting levels of phosphorus. Appl Soil Ecol 36: 205-215.
- Faye A, Dalpé Y, Ndung’u-Magiroi K, Jefwa J, Ndoye I, et al. (2013) Evaluation of commercial arbuscular mycorrhizal inoculants. Can J Plant Sci 93: 1201-1208.
- Miller MH, McGonigle TP, Addy HD (1995) Functional ecology of vesicular arbuscular mycorrhizas as influenced by phosphate fertilization and tillage in an agricultural ecosystem. Crit Rev Biotechnol 15: 241-255.
- Faggioli VS, Cabello MN, Grilli G, Vasar M, Covacevich F, et al. (2019) Root colonizing and soil borne communities of arbuscular mycorrhizal fungi differ among soybean fields with contrasting historical land use. Agric Ecosyst Environ 269: 174-182.
- Abbott LK, Robson AD (1982) The role of vesicular arbuscular mycorrhizal fungi in agriculture and the selection of fungi for inoculation. Aust. J Agric Res 33(2): 389-408.
- Ortas I, Rafique M, Ahmed IAM (2017) Application of Arbuscular mycorrhizal fungi into agriculture. In: Q. S. Wu (ed) Arbuscular mycorrhizas and stress tolerance of plants.
- Jacott CN, Murray JD, Ridout CJ (2017) Trade-Offs in Arbuscular Mycorrhizal Symbiosis: Disease Resistance, Growth Responses and Perspectives for Crop Breeding.
- Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320: 37-77.
- Finlay RD (2008) Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium. J Exp Bot 59(5): 1115-1126.
- Jaiswal DK, Verma JP, Prakash S, Meena VS, Meena RS (2016) Potassium as an important plant nutrient in sustainable agriculture: a state of the art. In: Meena VS, Maurya, BR, Verma, JP, Meena RS (Eds.), Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi: pp. 21-29.
- Kumar A, Maurya BR, Raghuwanshi R, Meena VS, Islam MT (2017) Co-inoculation with Enterobacter and Rhizobacteria on yield and nutrient uptake by wheat (Triticum aestivum ) in the alluvial soil under indo-gangetic plain of India. J Plant Growth Regul.
- Garg N, Chandel S (2010) Arbuscular mycorrhizal networks: process and functions. A review. Agron Sustain Dev 30: 581-599.
- Yadav BK, Sidhu AS (2016) Dynamics of potassium and their bioavailability for plant nutrition. In Potassium solubilizing microorganisms for sustainable agriculture pp. 187-201.
- Taber RA, Trappe JM (1982) Vesicular-arbuscular mycorrhiza in rhizomes, scale-like leaves, roots, and xylem of ginger. Mycol 74(1): 156-161.
- Lone R, Alaklabi A, Malik JA, Koul KK (2020) Mycorrhizal influence on storage metabolites and mineral nutrition in seed propagated potato (Solanum tuberosum) plant. Journal of Plant Nutrition 43(14): 2164-2175.
- Tairo EV, Ndakidemi PA (2013) Possible benefits of rhizobial inoculation and phosphorus supplementation on nutrition, growth and economic sustainability in grain legumes. American J Res Comm 1(12): 532-556.
- Balemi T, Negisho K (2012) Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: a review. J. Soil Sci. Plant Nutri 12(3): 547-562.
- Akiyama K, Matsuzaki KI, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435(7043): 824-827.
- Besserer A, Puech-Pagès V, Kiefer P, Gomez-Roldan V, Jauneau, A, Roy S, et al. (2006) Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol 4(7): 1239-1247.
- Kosuta S, Chabaud M, Lougnon G, Gough C, Dénarié J, et al. (2003) A Diffusible Factor from Arbuscular Mycorrhizal Fungi Induces Symbiosis-Specific MtENOD11 Expression in Roots of Medicago truncatula. Plant physiol 131(3): 952-962.
- Yang SY, Grønlund M, Jakobsen I, Grotemeyer MS, Rentsch D, et al. (2012) Nonredundant regulation of rice arbuscular mycorrhizal symbiosis by two members of the PHOSPHATE TRANSPORTER1 gene family. The Plant Cell 24(10): 4236-4251.
- Smith SE. Smith FA (2011) Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol 62: 227-250.
- Barea JM, Azcon-Aguilar C (1983) Mycorrhizas and their significance in nodulating nitrogen-fixing plants. Adv Agrono 36: 1-54.
- Azcón, R, Rubio R Barea JM (1991) Selective interactions between different species of mycorrhizal fungi and Rhizobium meliloti strains, and their effects on growth, N2‐fixation (15N) and nutrition of Medicago sativa L. New Phytol 117(3): 399-404.
- Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycor 11(1): 3-42.
- Ruiz-Lozano JM, Azcón R.Gomez M (1995) Effects of arbuscular-mycorrhizal glomus species on drought tolerance: physiological and nutritional plant responses. Appl Environ. Microbiol 61(2): 456-460.
- Nelsen CE, Safir G (1982) Increased drought tolerance of mycorrhizal onion plants caused by improved phosphorus nutrition. Planta 154(5): 407-413.
- Feng G, Zhang F, Li X, Tian C, Tang C, et al. (2002) Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycor 12(4):185-190.
- Al-Karaki GN, Al-Raddad A (1997) Effects of arbuscular mycorrhizal fungi and drought stress on growth and nutrient uptake of two wheat genotypes differing in drought resistance. Mycor 7(2): 83-88.
- Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann. Bot 104(7): 1263-1280.
- Giri B, Kapoor R, Mukerji KG (2007) Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Micro Ecol 54(4): 753-760.
- Kariman K, Barker SJ, Finnegan PM, Tibbett M (2014) Ecto-and arbuscular mycorrhizal symbiosis can induce tolerance to toxic pulses of phosphorus in jarrah (Eucalyptus marginata) seedlings. Mycorrhiza 24(7): 501-509.
- Bødker L, Kjøller R, Kristensen K, Rosendahl S (2002) Interactions between indigenous arbuscular mycorrhizal fungi and Aphanomyces euteiches in field-grown pea. Mycor 12(1): 7-12.
- Pozo MJ, Azcón-Aguilar C (2007) Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol 10(4): 393-398.
- Cordier C, Pozo MJ, Barea JM, Gianinazzi, S, Gianinazzi-Pearson V (1998) Cell defense responses associated with localized and systemic resistance to Phytophthora parasitica induced in tomato by an arbuscular mycorrhizal fungus. Mol. Plant-Microbe Interact 11(10): 1017-1028.
- Kaeppler SM, Parke JL, Mueller SM, Senior L, Stuber C, et al. (2000) Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci 40(2): 358-364.
- Mark GL, Cassells AC (1996) Genotype-dependence in the interaction between Glomus fistulosum, Phytophthora fragariae and the wild strawberry (Fragaria vesca). Plant soil 185(2): 233-239.
- Haynes RJ (1982) Effects of liming on phosphate availability in acid soils. Plant soil 68(3): 289-308.
- Kumar M, Yadav V, Kumar H, Sharma R, Singh A, et al. (2011) Piriformospora indica enhances plant growth by transferring phosphate. Plant Signal Behav 6(5): 723-725.
- Grace EJ, Cotsaftis O, Tester M, Smith FA, Smith SE (2009) Arbuscular mycorrhizal inhibition of growth in barley cannot be attributed to extent of colonization, fungal phosphorus uptake or effects on expression of plant phosphate transporter genes. New Phytol 181(4): 938-949.
- Li H, Smith SE, Holloway RE, Zhu Y, Smith FA (2006) Arbuscular mycorrhizal fungi contribute to phosphorus uptake by wheat grown in a phosphorus‐fixing soil even in the absence of positive growth responses. New Phytol 172(3): 536-543.
- Jones JD, Dangl JL (2006) The plant immune system. Nature 444(7117):323-329.
- Tisserant E, Kohler A, Dozolme‐Seddas P, Balestrini R, Benabdellah K, et al. (2012) The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol 193(3): 755-769.
- Sacristán D, González Guzmán A, Barrón V, Torrent J, Del Campillo MC (2019) Phosphorus-induced zinc deficiency in wheat pot-grown on noncalcareous and calcareous soils of different properties. Archiv Agron Soil Sci 65(2): 208-223.
- Rahmatullah, Torrent J (2000) Phosphorus dynamics and uptake by wheat in a model calcite-ferrihydrite system. Soil Sci 165(10): 803-812.
- Amanullah I, Inamullah X, Mona S, Alwahibi Elshikh S, Alkahtani J, et al. (2020) Phosphorous and Zinc Fertilization improve Zinc biofortification in grains and straw of coarse vs fine rice genotypes. Agron 10: 1155.
- Amanullah SA, Iqbal A, Fahad S (2016) Foliar phosphorus and zinc application improve growth and productivity of maize (Zea mays L.) under moisture stress conditions in semi-arid climates. J Microb Biochem Technol 8(5): 433-439.
- Haldar M,Mandal LN (1981) Effect of phosphorus and zinc on the growth and phosphorus, zinc, copper, iron and manganese nutrition of rice. Plant Soil 59(3): 415-425.
- Cakmak I, Marschner H (1987) Mechanism of phosphorus‐induced zinc deficiency in cotton. III. Changes in physiological availability of zinc in plants. Physiologia Plantarum 70: 13-20.
- Bhardwaj G, Sharma U, Brar PS (2019) A Review on Interactive Effects of Phosphorous, Zinc and Mycorrhiza in Soil and Plant. Int. J. Curr. Microbiol. App Sci 8(04): 2525-2530.
- Nguyen TD, Cavagnaro TR, Watts-Williams SJ (2019) The effects of soil phosphorus and zinc availability on plant responses to mycorrhizal fungi: a physiological and molecular assessment. Scientific Reports 9: 14880.
- Plassard C,Dell B (2010) Phosphorus nutrition of mycorrhizal trees. Tree physiology 30(9):1129-1139.
- Smith SE. Smith FA (2012) Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycol 104(1): 1-13.
- Bolan NS (1991) A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant soil 134(2): 189-207.
- Sindhu SS, Parmar P, Phour M, Sehrawat A (2016) Potassium-solubilizing microorganisms (KSMs) and its effect on plant growth improvement. In Potassium solubilizing microorganisms for sustainable agriculture pp. 171-185.
- Almagrabi OA, Abdelmoneim TS (2012) Using of Arbuscular mycorrhizal fungi to reduce the deficiency effect of phosphorous fertilization on maize plants (Zea mays). Life Sci J 9(4): 1648-1654.
- Liu A, Hamel C, Hamilton RI, Smith DL (2000) Mycorrhizae formation and nutrient uptake of new corn (Zea mays L.) hybrids with extreme canopy and leaf architecture as influenced by soil N and P levels. Plant Soil 221(2): 157-166.
- Balzergue C, Puech-Pagès V, Bécard G,Rochange SF (2011) The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events. J Exp Bot 62(3):1049-1060.
- Nagy R, Drissner D, Amrhein N, Jakobsen I, Bucher M (2009) Mycorrhizal phosphate uptake pathway in tomato is phosphorus‐repressible and transcriptionally regulated. New Phytol 181(4): 950-959.
- Asmah AE (1995) Effect of phosphorus source and rate of application on VAM fungal infection and growth of maize (Zea mays ). Mycor 5(3): 223-228.
- Allen JW, Shachar-Hill Y (2009) Sulfur transfer through an arbuscular mycorrhiza. Plant Physiol 149: 549-560.
- Amanullah I, Inamullah X (2016) Dry matter partitioning and harvest index differ in rice genotypes with variable rates of phosphorus and zinc nutrition. Rice Sci 23(2): 78-87.
- Karasawa T (2004) Arbuscular mycorrhizal associations and interactions in temperate cropping systems. Res Bull Natl Agric Res Cent 179: 1-71.
- Ortas I, Rafique M, Ahmed IAM (2017) Application of Arbuscular mycorrhizal fungi into agriculture. In: QS Wu (Eds) Arbuscular mycorrhizas and stress tolerance of plants.
- Sanders D (2008) Zinc biofortification of cereals: problems and solutions. Trends.
- Sieh D, Watanabe M, Devers EA, Brueckner F, Hoefgen R, et al. (2013) The arbuscular mycorrhizal symbiosis influences sulfur starvation responses of Medicago truncatula. New Phytol 197(2): 606-616.
- Smith FA, Grace EJ, Smith SE (2009) More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol 182(2): 347-358.
- Smith, S E, Read DJ (2010) Mycorrhizal symbiosis. Academic press.
- Watts-Williams SJ, Smith FA, McLaughlin MJ, Patti AF, et al. (2015) How important is the mycorrhizal pathway for plant Zn uptake? Plant Soil 390: 157-166.

 Review Article
Review Article