Abstract
The aim of this study was to evaluate the safety of the extracts of poria cocos, Tumulosic acid and Polyporenic Acid C, by acute oral toxicity test and micronucleus test for estimating the gene toxicity on ICR male mice. Our results revealed that there were no abnormal symptoms found in mice fed with Tumulosic acid (25 mg/ kg- BW) or Polyporenic Acid C (10 mg/ kg-BW) during the 14 day experimental period. The hematological analysis was within normal range and no obvious genotoxicity in micronucleus test of mice fed with indicated dosages of Tumulosic acid or Polyporenic Acid C compounds, and there was no significant difference among tissue weights of heart, liver, lung and kidney within each groups. However, we found that feeding of Tumulosic acid or Polyporenic Acid C resulted in decreased weight of immune organs, such as spleen and thymus. The histopathology demonstrated a mild lymphoid apoptosis in both the thymus and spleen. Collected data indicated that there were no toxic effect on murine fed with Tumulosic acid or Polyporenic Acid C compound in 14 days, but there might be immunotoxicity. Based on these findings, there seems to be a need for further studies.
Keywords: Poria Cocos; Triterpenoid; Acute Oral Toxicity Test; Immunotoxicity
Abbreviations: PCP: Poria Cocos Polysaccharide; USEPA: United States Environmental Protection Agency; HPLC: High-Performance Liquid Chromatography; TA: Tumulosic Acid; PC: Polyporenic Acid C; SPF: Specific Pathogen Free; MN: Micronuclei; RET: Reticulocyte; CD: Cluster of Differentiation; CBC: Complete Blood Count; RBC: Red Blood Cell Count; WBC: White Blood Cell Count; HGB: Hemoglobin; HCT: Hematocrit; MCH: Mean Corpuscular Hemoglobin; MCV: Mean Corpuscular Volume; MCHC: Mean Corpuscular Hemoglobin Concentration; PLT: Platelet Count; MPV: Mean Platelet Volume; RDW: Red Cell Distribution Width; PDW: Platelet Distribution Width; PVR: Platelet Volume Ratio; NEU: Neutrophil Count; LYM: Lymphocyte Count; EOSI: Eosinophil; BASO: Basophil; LUC: Large and Unstained Leukocyte; TDT: Terminal Deoxynucleotidyl Transferase
Introduction
Poria cocos (Schw.) Wolfis is a type of fungus which belongs to fungi kingdom, Basidiomycota, Basidiomycetes, Polyporales, Fomitopsidaceae and genus Wolfiporia. Poria cocos (Schw.) Wolfis is a polyporaceae fungi dried sclerotia, often a commensal found on the roots of pine trees. Spherical in shape, externally black-brown in color, while internally it is pink or white in color. Poria cocos (Schw.) Wolfis has multiple physiologic activities like regulating serum fatty acid [1,2], anti-oxidative and anti-inflammatory [3,4], anti-carcinogenic [5-7], and immunoregulating effects [8-10]. Poria cocos (Schw.) Wolfis has been studied for the efficacy of its ingredients such as polysaccharides, sterols and other components. Its main polysaccharide part is β-pachyman. Study has suggested that Poria Cocos Polysaccharide (PCP) stimulated dendritic cell maturation, cytokine secretion, antigen presentation, crosspresentation and promoted Th1-dominant response [11]. PCP has been used as an antitumour drug in China through stimulating the expression of apoptosis‐related genes and regulating the activities of TPK and PTPP in cancer cells [12]. While its sterols is mostly tetracyclic triterpenoids classified as lanostane and secolanostane, including pachymic acid, tumulosic acid, polyporenic acid C, ebricoic acid, and poricoic acid. The other functional components of Poria cocos (Schw.) Wolfis are ergosterol, protein, lecithin, adenine, choline, histidine, fatty acid, and other inorganic elements. The main component regulating biologic activities are polysaccharide [13,14] and triterpenoid.
Throughout the history of Chinese medicine, Poria cocos has been recommended in numerous publications for being an effective cough expectorant, diuretic, etc. However these materials lack scientific evidence. There is doubt that an excess intake of Poria cocos may cause toxicity. In order to clarify these questions, we adopt the strategies of acute oral toxicity test following both Taiwan Ministry of Health and the United States Environmental Protection Agency (USEPA) established Health Effects Test Guideline. We used the ICR male mice as our study animal and fed it with Triterpenoidrich extracts derived from Poria cocos in a safe experimental assessment. We hope the result of this study can in the future provide more information in developing functional products from triterpenoid of Poria cocos.
Materials and Methods
Preparation of Materials
The Poria cocos (Schw.) Wolfis extracts was provided by Sinphar Pharmaceutical Corporation (Taipei, Taiwan). Extracts of triterpenoids was prepared by the following method: one kilogram of the commercially available Poria cocos (Schw.) was powderized and dissolved in 10 liters of alcohol. We got 10 grams of the extracts after 3 consecutive extraction with alcohol. The extracts then undergo separation by column chromatography. The extract was further purified by column chromatography with dichloromethanemethanol (96: 3 or 96: 4). The purified triterpenoid was analyzed by High-Performance Liquid Chromatography (HPLC), and the purity is more than 95% with the following components in sequence: 100 mg dehydropachymic acid and pachymic acid;200 mg Tumulosic Acid (TA);60 mg polyporenic acid C (PC);20 mg 3-epidehydrotumulosic acid and other smaller amount of lanosterol compounds. Since the extract is a fat-soluble sterol, the test powder is dissolved with small amount of 75% alcohol, with distilled water added and quantified to an appropriate concentration.
Experimental Animal
Six to eight weeks old ICR male mice were purchased from Lasco Biotechnology Corporation, limited (Yilan, Taiwan) and are Specific Pathogen Free (SPF), mice excluded pathogens are: PVM, revirus 3, Sendai virus, LCMV, hantavirus, TMEV, mouse adenovirus, minute virus of mice, Ectromelia, MHV, Mycoplasma pulmonis, Bordetella bronchiseptica, Clostridium piliforme, Corynebacterium kutscheri, Salmonella spp., Myobia musculi, Aspiculuris tetraptera, Syphacia obvekata, Syphacia muris, Rodentolepsis nana and Rodentolepsis diminuta). ICR male mice are raised in sterilized, individually ventilated cage (IVC;SLIM LIMETM) at the animal center of the department of nutrition, Fu Jen University. The mice were provided with sterilized pad (hygienic animal bedding, D-73484, Germany) and were freely fed on chow diet (laboratory rodent diet 2001, PMI Nutrition international, Inc., USA) and distilled water. The animal center room temperature was maintained at 25 ± 3℃ with a relative humidity of 50~80% and a 12 hourly light & dark illumination cycle.
Experimental Design
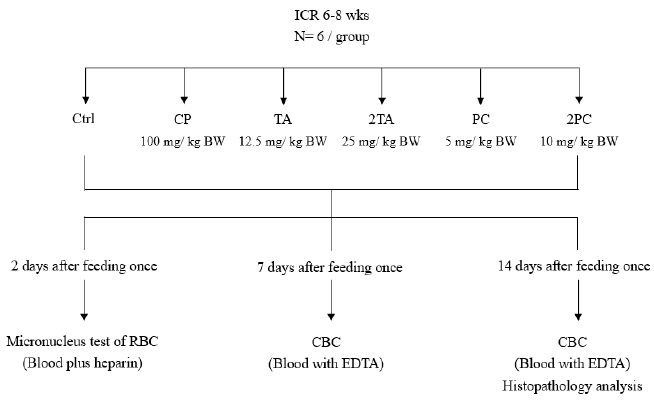
Our study used acute oral toxicity test conducted by the Ministry of Health and the United State Environmental Protection Agency (USEPA) established Health Effects Test Guideline; Acute Oral Toxicity; Harmonized Test Guideline. The experimental mice were divided into 6 groups with each group having a total of 6 mice. The first group was assigned as the control group (Ctrl group), which we also considered as the negative control group. The second group was the intraperitoneally injected chemotherapy group (cyclophosphamide, CP, 100mg/kg-BW, i.p). The last 4 groups were the single orally fed mice which are: TA group (12.5 mg/kg-BW), 2TA group (25 mg/kg-BW), PC group (5 mg/kg-BW) and the 2PC group (10 mg/kg-BW) (Figure 1). Triterpenoid extract of Poria cocos is a fat-soluble sterol, so we first use small amount of 75% alcohol to dissolve TA and PC. Then we used distilled water to titrate to the appropriate concentration and fed the mouse through feeding tube. The mice were observed daily after feeding and weighed once a week. Blood sample from the animal’s facial vein were done every 2nd, 7th and 14th day after treatment and the blood were collected for micronuclei test and hematologic analysis. On the 14th day after treatment, the mice were undergo asphyxiation with carbon dioxide.
Micronuclei Test
Micronuclei (MN) test is a good indicator of chromosomal damage [15]. Abnormal micronuclei formation occurs when the nuclei of cells undergoing mitotic division experiences damage or destruction. The formed micronuclei resides and accumulates within the nucleus of the cell; thus, causing genotoxicity, Using the peripheral reticulocyte (RET) with specific transferrin receptor cluster of differentiation (CD) 71, the red cells were marked with the anti-CD 71-FITC antibody (fluorescein isothiocyanate) and propidium iiodide. The marked cells exhibited the red fluorescence (PI). Through flow cytometry the cells, which exhibited the red fluorescence, were measured. The measurement, in percentage, compares the amount of the abnormal red cells having micronuclei to the normal red cells throughout the body [16]. The blood of the mice were collected 48 hours after feeding test substance. In accordance to the steps in the manual of Micro-nucleation detection kit (FC008, Gene Research Laboratory company, Limited, purchased from Jingjing Biochemical Technology Company Limited, Taipei, Taiwan), the extracted blood were tested for the micronuclei ratio together with flow cytometry (Cyflow, Partech, Germany) and WinMDI 2.8 analysis software, we then can observed the distribution of micronuclei in the red cell.
Hematologic Test
The extracted blood from the facial vein of the mice were placed in an anticoagulant EDTA-containing (C10H16N2O8 syringe, Vacutainer, NJ, USA) test tube. At room temperature, the blood immediately underwent an analysis by hemocytometery (ADVIA® 2120 Hematology System, Germany;National Laboratory Animal Center Foundation). Complete blood count (CBC) included: Red Blood Cell Count (RBC), white blood cell count (WBC), Hemoglobin (HGB), Hematocrit (HCT), Mean Corpuscular Hemoglobin (MCH), Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin Concentration (MCHC), Platelet count (PLT), Mean Platelet Volume (MPV), Red cell Distribution Width (RDW), Platelet Distribution Width (PDW), Platelet Volume Ratio (PCT), Neutrophil count (NEU), Lymphocyte Count (LYM), Monocyte (Mono), Eosinophil (EOSI), Basophil (BASO), and Large and Unstained Leukocyte (LUC).
Organ Pathologic Examination
On the 14th day, the ICR male mice were asphyxiated with carbon dioxide and its organs such as the heart, liver, spleen, kidney and thymus were obtained. Each organ was weighed and compared with the body weight of the mice. The relative weight percentage of each organ was expressed as the weight of each organ over the body weight of the mice. The organs were immersed in 10% formalin solution (mixing 1 ml formaldehyde solution in 9 mL PBS for dilution) and fixed for 24 hours. The tissues were cut into 0.3-0.4 cm slices and were embedded in paraffin wax. After the tissue block was prepared, a 3 μm thick ribbon is made. Using Hematoxylin & Eosin (H & E) staining, this ribbon was then used for the microscopic examination of the tissue organs for histopathologic changes.
Statistical Analysis
The experimental results were expressed in mean ± SD. Student’s t test was used to analyze the differences in the micronucleic analysis between each experimental group from the control and the CP group. P < 0.05 signifies a significant difference between the compared groups which was shown in the diagram presented.
Results
Effect of triterpenoids TA and PC on the body weight of ICR mice during oral acute toxicity test
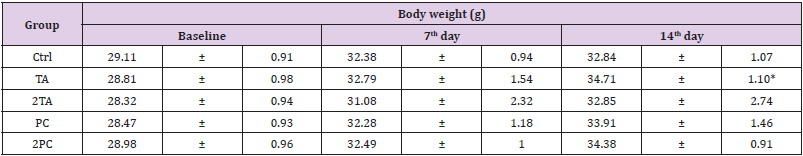
To understand the effect of high dose test substance on the body weight of the ICR mice, the mice were weighed on the 7th and 14th day after feeding. The results in comparison to the control group showed no significant difference in body weight among those mice fed with high dose TA and PC on the 7th day after feeding. The same goes with those mice fed with high dose PC after the 14th day of feeding. However, those mice fed with high dose TA after 14th day after feeding showed a significantly higher body weight compared to the control group on the 14th day after feeding (Table 1).
Table 1: The effects of extra-high dosages of TA and PC on body weight change of ICR male mice in acute oral toxicity test.
Note: The values were presented as mean ± SD. Each group contains 6 mice. Value having different superscripts are significantly different from baseline by Student’s t-test (*p<0.05).
There is no effect on the red blood cells’ micronuclei test of the ICR mice administered with Triterpenoid TA and PC.
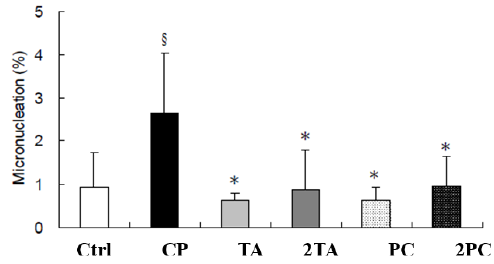
On the second day after high dose TA or PC tube feeding, blood examinations for the micronuclei analysis was done on the ICR male mice. The anti-transferrin receptor CD71 antibody marked red blood cells were stained with the FITC fluorescent dye and PI. Through this, we were able to count the number of micronucleicontaining red blood cells; thus, obtaining its percentage with the whole blood [15]. The proportion of micronuclei in the red blood cells were low. However, the percentage of micronuclei in the blood of the CP treated group, which was designated as the positive control, was significantly higher than the treated groups (p<0.05) as shown in Figure 2. In comparison to the negative control, the treated groups did not have significant difference in the micronuclei test. The results of both the negative and positive controls enforce that the tests performed were valid. The results indicate that high dose TA and PC did not show any significant genotoxicity in the Acute Oral Toxicity test (Figure 2).
Figure 2: In vivo genotoxicity of ICR mice fed with or without TA and PC. In vivo genotoxicity were represented as the percentage of micro-nucleation in the erythrocytes of whole blood compared to the control group. Cyclophosphamide (CP, 100 mg/ kg- BW) was used as the chemical of positive control. §Means statistical significance compared with the control group by Student’s t test. (p< 0.05). *Means statistical significance compared with the CP group by Student’s t test. (p< 0.05).
Effect of Triterpenoid TA and PC on blood cells of ICR mice
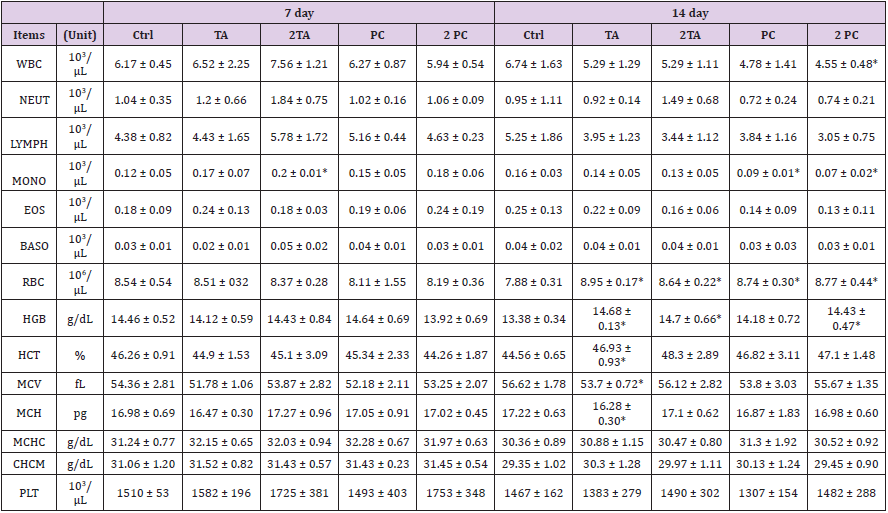
Whole blood was collected on the 7th and 14th day after feeding high dose of Poria cocos extracts (TA or PC). The blood collected were examined by the blood cell counter. The results showed the mice, which were fed high dose 2PC, on the 14th day, had a significantly low white blood cell count compared to the CP group. Its effect on the differential count of the white blood cells showed the mice fed 2TA on the 7th day had higher number of mononuclear cells compared to the CP group. The total number of mononuclear cells were significantly decreased in the PC and 2PC group on the 14th day after feeding. Basophils increased significantly on the 14th day after feeding high dose TA or PC. On the other hand, the result of the blood analysis (red blood cells) after the 14th day of feeding were as follows: all treated groups had Red Blood Count (RBC) significantly higher than the CP group; TA, 2TA and 2PC group had significantly higher HGB; TA group has significantly higher HCT, while MCV and MCH were significantly lower than the control group. Although there were many significant differences among the groups, the red blood cell indices were still within the normal range (Table 2). (8-week-old mouse blood biochemical data and reference values established by Dr. Liang Chong-Tiang’s reference data and from ADVIA2120 blood cell count reference).
Table 2: Hematological changes of ICR mice fed with or without TA and PC in acute oral toxicity test.
1Data were represented as mean ± SD (n=6).
2*Means statistical significance compared with Ctrl group by Student’s t-test (p<0.05)
Effect on the Weight and Histopathology of the Internal Organs of the ICR mice
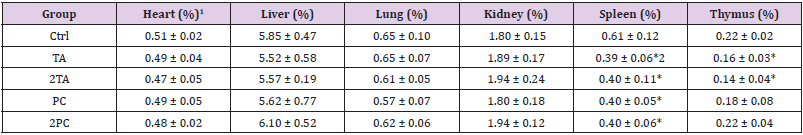
The mice were subjected to asphyxiation on the 14th day after feeding single dose or double dose of TA and PC. The organs such as the heart, liver, lung, kidney, spleen and thymus were removed and weighed. The percentage of relative weight of each organ is expressed as the ratio of the weight of each organ to the final body weight on the 14th day. The results showed no significant difference in weight of the heart, liver, lung and kidney of the control groups and treated groups. However the immune related organs, such as the spleen & thymus of those mice fed with TA, 2TA, PC and 2PC have significantly lower organ weight than the control group. It was presumed that the high dose of TA and TC in the oral acute toxicity study can have toxic effect on the immune related organs (Table 3).
Table 3: Hearing assessment at various time intervals.
1Organ weight (%) = [ Organ weight (g)/ final body weight (g) ] X 100.
2The values were presented as mean ± SD. Each group contains 6 mice. Values having different superscripts are significantly different from control group by Student’s t-test. (*p<0.05).
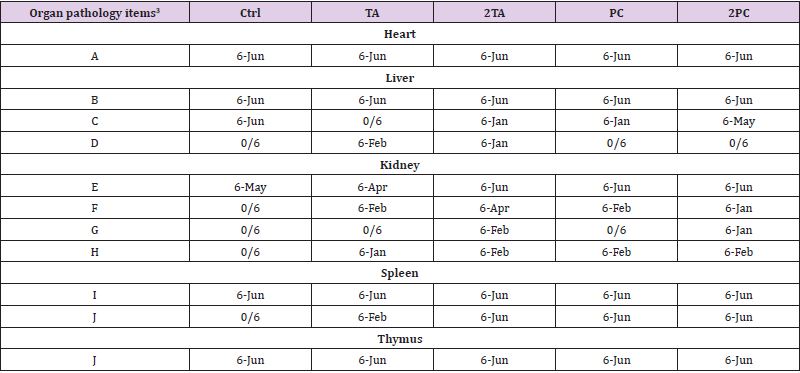
The mice fed with high dose of TA and TC were asphyxiated on the 14th day and its heart, liver, kidney, spleen and thymus were removed. These organs were stained with H&E and were examined under optical microscope for histopathologic changes. Results showed the following pathologic findings: A. myocardial fiber vacuolization; B. hepatocyte hypertrophy; C. hepatocyte vacuolization (glycogen or fat infiltration); D. liver cell necrosis; E. renal tubule epithelial cell basophilicity; F. renal tubule epithelial cell expansion and vacuolization; G. renal tubular cell hypertrophy and cystic tubule formation; H. renal Interstitial infiltration of mononuclear cells; I. splenic hematopoiesis; J. lymphocyte apoptosis. The above observed pathologic changes were organized according to the incidence of pathological findings (Table 4).
Table 4: Overview of incidence rate of histopathological findings of ICR mice fed with or without TA and PC in acute oral toxicity test1,2.
Note: 1These organs were stained with H&E and were examined under optical microscopy for histopathologic changes.
2The data were represented as: the number of specific organs with pathologic changes/the whole number of total observed organs.
3Organ pathology items including
(A) Sarcoplasmic vacuolation, myocardium;
(B) Karyomegaly, cytomegaly with distinct nucleoli;
(C) Hepatic cells vacuolation (glycogen or fatty infiltration);
(D) Hepatic cells necrosis;
(E) Renal tubules epithelium, basophilic;
(F) Dilated and vacuolated epithelium, renal cortex;
(G) Renal tubular cells hypertrophy and cyst;
(H) Mononuclear cells infiltration;
(I) Extramedullary hematopoiesis;
(J) Lymphoid apoptosis.
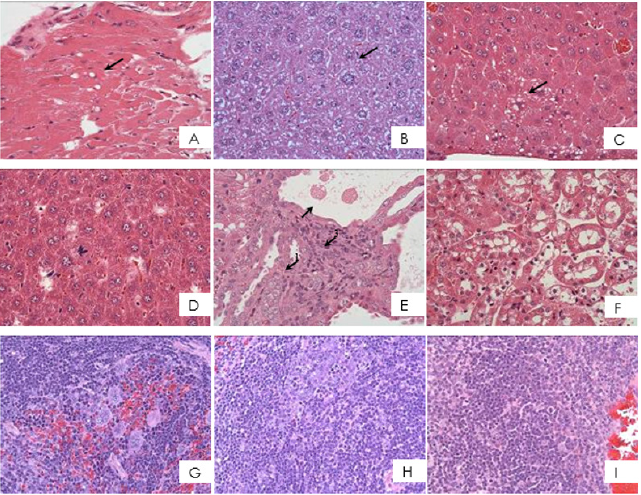
The result of the heart biopsy showed the control group and the treated groups were found to have vacuolization of myocardial fibers. The control group and the experimental group were both found to have hepatocellular hypertrophy but there were no significant difference. The liver, an organ with regenerative capacity, was expected to have an observed microscopic difference in cell size. The treated groups have a slight vacuolization of hepatocytes and liver cell necrosis, however the number of observed animals is small. That is why, compared to the control group, it is not considered a significant difference and it is speculated that the difference may be due to the individuality of the mouse and background lesions. It is observed that the kidney had slightly basophilic changes of renal tubular epithelial cells, renal cortical epithelial cell expansion and vacuolization, tubular cell hypertrophy, tubular cystic changes and mononuclear cell infiltration. There were mild and focal pathological lesions and were only observed in only a few animals of the experimental groups. In comparison to the changes of the control group, no significant difference was attained. Thus, suggesting that the test substance does not cause any toxic effects on the kidney. While in the immune related organs such as spleen and thymus, results showed that the control group and treated group have extramedullary hematopoiesis phenomenon in the spleen. The 2TA, PC, and 2PC groups have a significant difference in lymphocyte apoptosis in the spleen as compared to the control group. In the thymus, both the control and experimental groups have a slight cellular apoptosis (Figure 3).
Figure 3: Histopathological findings of ICR mice fed with or without TA and PC in acute oral toxicity test.
A. Sarcoplasmic vacuolation myocardium, heart (arrow)
B. Karyomegaly, cytomegaly with distinct nucleoli, liver (arrow)
C. Hepatic cells vacuolation (arrow)
D. Hepatic cells necrosis, focal (arrow)
E. Renal tubules epithelium, basophilic, focal (arrow1), renal tubular cells hypertrophy and cyst (arrow2), mononuclear cells infiltration (arrow3)
F. Dilated and vacuolated epithelium, renal cortex
G. Extramedullary hematopoiesis
H. Lymphoid apoptosis, mild to slight, spleen
I. Lymphoid apoptosis, mild, thymus (H&E stain, 400X).
Discussion
Poria cocos, Fu-Ling, is an oriental fungus and has been widely used as a Chinese traditional herbal medicine for centuries. Compendium of Materia Medica recorded that Poria cocos has many beneficial medical effects and indications for use in the traditional Chinese medicine. These include promoting urination, to invigorate the spleen function (i.e., digestive function), and to calm the mind [17]. Poria is not only used as component of traditional Chinese herbal medicine, it is also a component of many nourishing soups due to its food and drug characteristics [18]. In recent years, the studies involving the physiological activity of Poria cocos has focused more on anticancer, antioxidant and anti-hyperlipidemia. Ukiya et al discovered that lanostane-type triterpenes components such as poricoic acid, tumulosic acid and polyporenic acid showed potent inhibitory effects on Epstein-Barr virus early antigen activation induced by the tumor promoter 12-0-tetradecanoyphorbol-13- acetate [18]. In addition, it has cytotoxic effect on human leukemia cell line HL-60 cells [19]. At the same time, some studies state that triterpene dehydroebriconic acid could prevent the growth of human gastric cancer cells, and the cells were halted at the G1 phase in the cell cycle [20].
Recently, there are many research about the safety evaluation of the natural foods, especially those plants extracted active ingredients using acute toxicity and mutagenicity test [21]. ICR mice were hybridized strains, and therefore safety evaluation tests often use ICR mice as an experimental animal model [21,22]. In this study of acute oral toxicity test we used ICR mice as an experimental model. Our results showed TA fed mice on the 14th day did have a significantly higher body weight than the control group. Previous study had shown Poria extract and purified lanostanes have an effect on human intestines and enhance the uptake of nutrients: absorption of glucose, amino acids, and vitamins (folic acid). Poria extract ingredient, particularly lanostane compounds, might help in improving and treating cachexia associated with nutrition and immunology [23]. TA and PC were purified from triterpenoid pure extracts of Poria Cocos. After the mice were fed on a single high dose of the test substance TA & PC, none of the test mice died during the 14 day observation. For the micronuclei test, intraperitoneal injection of 100 mg/Kg-BW of chemotherapy drug, cyclophosphamide, served as positive control group. We then observed that feeding TA and PC did not increase the level of micronucleus in the blood of mice. The treated group has significantly lower micronucleus than the positive control group. There were no difference in the percentage of micronucleus results compared with the control group. Therefore, this confirmed that the addition of TA and PC did not cause any genotoxicity in mice.
The results showed the mice which were fed with high dose 2PC on the 14th day had a significantly low white blood cell count compared to the control group. Its effect on the differential count of the white blood cells showed that on the 7th day of the mice fed with 2TA had higher mononuclear cell compared to the control group. The total number of mononuclear cells was significantly decreased in the PC and 2PC group 14 days after feeding. While polysaccharide fraction from Poria cocos was found to have growth-inhibitory and differentiation-inducing activities on human leukemic cells, U937 and HL-60 through elevated cytokines of IFN-γ and TNF-α [19]. All test groups had Red Blood Count (RBC) significantly higher than those control group; TA, 2TA and 2PC group had significantly higher HGB, TA group has significantly higher HCT, while MCV and MCH were significantly lower than the control group. Triterpene carboxylic acids isolated from the methanol extract of Hoelen (sclederma of Poria cocos Wolf) were found to have has the scavenging effect on free-radicals and inhibitory activities against AAPH (2, 2’-azobis (2-amidinopropane) hydrochloride)-induced lysis of red blood cells [24]. Although many significant differences in the red blood cell indices among the groups but all data are still within the normal range.
High-dose TA and PC did not affect the weight of vital organs like the heart, liver, lung and kidney. Although there are some histopathological changes on heat, liver, kidney and thymus, these lesions are focal and minor with no serious nor widespread lesions. These changes has no significant difference compared with the control group. However, the histopathological findings in the spleen among the 2TA, PC and 2PC groups showed significant cellular apoptosis. Morphologically, apoptosis has cell shrinkage, chromatin condensation and DNA fragments forming apoptotic bodies [25]. Kikuchi & others suggested that apoptosis-inducing activities of triterpene acids from Poria cocos is via the mitochondrial pathway mostly by translocation of apoptosis-inducing factor, independent from the caspase pathway in A549 [26]. Reduced weight of the spleen in Table 3 and the statistical incidence of the pathologic lesion of spleen’s lymphocyte cellular apoptosis in Table 4 were consistent. It was speculated that although the pathologic weight change of the spleen among the study group was seen, it was based only on H & E staining, by which H & E staining is not apoptosisspecific staining method. Therefore, these morphologic changes are only preliminary result. In the future, cell apoptosis-specific staining, such as terminal deoxynucleotidyl transferase (TdT) - mediated dUTP-biotin nick end-labeling (TUNEL) [27] can be used for further analysis.
In summary, given single dose of 25 mg of TA per kilogram of body weight and 10 mg of PC per kilogram of body weight did not cause any toxic reaction to ICR male mice. Our finding is same with Zhang et al experiment, rats fed SD 10,000 mg / kg-BW Bamboo mushroom triterpenoids does not cause toxic effects [21]. In the future, we plan to use a much higher dosage and investigate whether it would cause any other toxic effects in the mice. In our study, our experimental dosage cause reduction in the weight of immune related organs like spleen and thymus. Histopathologic changes in spleen and thymus showed lymphocyte apoptosis. Therefore, whether high doses of TA and PC will cause immunosuppressive effects, further study is needed.
References
- Kim BJ, Kim K, Park WH, Ko JH, Lee YC (2003) A water-extract of the Korean traditional formulation Geiji-Bokryung-Hwan reduces atherosclerosis and hypercholesteremia in cholesterol-fed rabbits. Int immunopharmacol 3(5): 723-734.
- Miao H, Zhao YH, Vaziri ND, Tang DD, Chen H, et al. (2016) Lipidomics biomarkers of diet-induced hyperlipidemia and its treatment with Poria cocos. J Agric Food Chem 64(4): 969-979.
- Schinella GR, Tournier HA, Prieto JM, Mordujovich BP, Ríos JL (2002) Antioxidant activity of anti-inflammatory plant extracts. Life Sci 70(9): 1023-1033.
- Li FF, Yuan Y, Liu Y, Wu QQ, Jiao R, et al. (2015) Pachymic acid protects H9c2 cardiomyocytes from lipopolysaccharide-induced inflammation and apoptosis by inhibiting the extracellular signal-regulated kinase 1/2 and p38 pathways. Mol Med Rep 12(2): 2807-2813.
- Gapter L, Wang Z, Glinski J, Ng KY (2005) Induction of apoptosis in prostate cancer cells by pachymic acid from Poria cocos. Biochem Biophys Res Commun 332(4): 1153-1161.
- Jeong JW, Lee WS, Go SI, Nagappan A, Baek JY, et al. (2015) Pachymic acid Induces apoptosis of EJ bladder cancer cells by DR5 up-regulation, ROS generation, modulation of Bcl-2 and IAP family members. Phytother Res 29(10): 1516-1524.
- Lin Y, Zhang L, Chen L, Jin Y, Zeng F, et al. (2004) Molecular mass and antitumor activities of sulfated derivatives of alpha-glucan from Poria cocos mycelia. Int J Biol Macromol 34(5): 289-294.
- Yu SJ, Tseng J (1996) Fu-Ling, a Chinese herbal drug, modulates cytokine secretion by human peripheral blood monocytes. Int J Immunopharmacol 18(1): 37-44.
- Lee KY, Jeon YJ (2003) Polysaccharide isolated from Poria cocos sclerotium induces NF-kappaB/Rel activation and iNOS expression in murine macrophages. Int Immunopharmacol 3: 1353-1362.
- Lu YT, Kuan YC, Chang HH, Sheu F (2014) Molecular cloning of a Poria cocos protein that activates Th1 immune response and allays Th2 cytokine and IgE production in a murine atopic dermatitis model. J Agric Food Chem 62(13): 2861-2871.
- Dong X, Li B, Yu B, Chen T, Hu Q, et al. (2021) Poria cocos polysaccharide induced Th1-type immune response to ovalbumin in mice. PLoS One 16(1): e0245207.
- Li X, He Y, Zeng P, Liu Y, Zhang M, et al. (2019) Molecular basis for Poria cocos muschroom polysaccharide used as an antitumour drug in China. Cell Mol Med 23(1): 4-20.
- Wang H, Mukerabigwi JF, Zhang Y, Han L, Jiayinaguli T (2015) In vivo immunological activity of carboxymethylated-sulfated(1→3)-β-D-glucan from sclerotium of Poria cocos. Int J Biol Macromol 79: 511-517.
- Wu Y, Li S, Li H, Zhao C, Ma H, et al. (2016) Effect of a polysaccharide from Poria cocos on humoral response in mice immunized by H1N1 influenza and HBsAg vaccines. Int J Biol Macromol 91: 248-257.
- Heddle JA, Cimino MC, Hayashi M, Romagna F, Shelby MD, et al. (1991) Micronuclei as an index of cytogenetic damage: past, present, and future. Environ Mol Mutagen 18(4): 277-291.
- Witt KL, Livanos E, Kissling GE, Torous DK, Caspary W, et al. (2008) Comparison of flow cytometry- and microscopy-based methods for measuring micronucleated reticulocyte frequencies in rodents treated with non-genotoxic and genotoxic chemicals. Mutat Res 649(1-2): 101-113.
- Bensky D, Clavey S, Stoger E (2004) Materia Medica (3rd ,). Eastland Press, Inc: Seattle.
- Ukiya M, Akihisa T, Tokuda H, Hirano M, Oshikubo M, et al. (2002) Inhibition of tumor-promoting effects by poricoic acids G and H and other lanostane-type triterpenes and cytotoxic activity of poricoic acids A and G from Poria cocos. J Nat Prod 65(4): 462-465.
- Chen YY, Chang HM (2004) Antiproliferative and differentiating effects of polysaccharide fraction from fu-ling (Poria cocos) on human leukemic U937 and HL-60 cells. Food Chem Toxicol 42(5): 759-769.
- Mizushina Y, Akihisa T, Ukiya M, Murakami C, Kuriyama I, et al. (2004) A novel DNA topoisomerase inhibitor: dehydroebriconic acid, one of the lanostane-type triterpene acids from Poria cocos. Cancer Sci 95(4): 356-360.
- Zhang Y, Wu X, Ren Y, Fu J, Zhang Y (2004) Safety evaluation of a triterpenoid-rich extract from bamboo shavings. Food Chem Toxicol 42(11): 1867-1875.
- De Boeck M, Van der Leede BJ, Van Goethem F, De Smedt A, Steemans M, et al. (2005) Flow cytometric analysis of micronucleated reticulocytes: Time- and dose-dependent response of known mutagens in mice, using multiple blood sampling. Environ Mol Mutagen 46(1): 30-42.
- (2014) Sinphar Tian-Li Pharmaceutical Co., Ltd.; Patent issued for use of Lanostane and Poria extract in treating Cachexia. AIDS Weekly, pp. 243.
- Sekiya N, Goto H, Shimada Y, Endo Y, Sakakibara I, et al. (2003) Inhibitory effects of triterpenes isolated from Hoelen on free radical-induced lysis of red blood cells. Phytother Res 17(2): 160-162.
- Cohen JJ (1999) Apoptosis: mechanisms of life and death in the immune system. J Allergy Clin Immunol 103(4): 548-554.
- Kikuchi T, Uchiyama E, Ukiya M, Tabata K, Kimura YT, et al. (2011) Cytotoxic and apoptosis-inducing activities of triterpene acids from Poria cocos. J Nat Prod 74(2): 137-144.
- Kumagai K, Yamaguchi R, Uchida K, Tateyama S (2004) Lymphoid apoptosis in acute canine distemper. J Vet Med Sci 66(2): 175-181.

 Research Article
Research Article