ABSTRACT
Keywords: Demyelination; Etanercept; Therapy;Tumor Necrosis Factor; Exacerbation
Introduction
Tumor necrosis factor (TNF) alpha is an immunomodulatory cytokine, implicated in mechanism of inflammatory and autoimmunologic diseases, like multiple sclerosis (MS), rheumatoid arthritis (RA) and Crohn disease [1]. At present five anti-TNF agents are in clinical use including soluble TNFR2 (etanercept) and four anti-TNF specific monoclonal antibodies [2]. Etanercept has been shown to interfere with TNF bioavailability and it is approved for treatment of rheumatoid arthritis, juvenile rheumatoid arthritis and psoriatic arthritis, plaque psoriasis and ankylosing spondylitis. Treatment with etanercept and other anti-TNF therapeutics was associated with increased risks for bacterial, viral and fungal infections, hemocytopenias, congestive heart failure and development of T-cell lymphomas. In addition it was observed that RA patients treated with anti-TNF therapy are at risk either to develop or exacerbate demyelination within CNS [3]. Here we report a case of a first demyelinating episode in RA patient during etanercept therapy and discuss the possible association between TNF inhibition and clinical demyelination.
Case Report
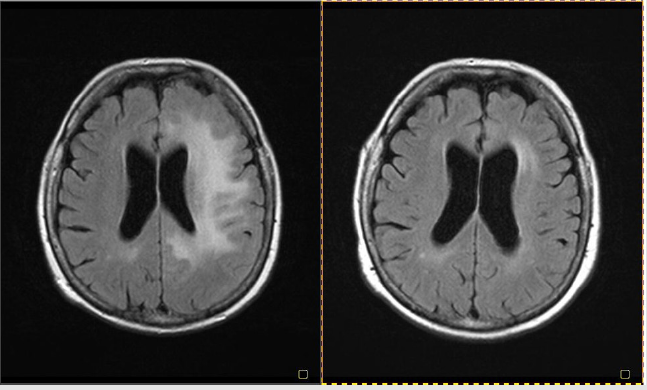
A 60-year-old Caucasian female, with a history of rheumatoid arthritis, has been treated with etanercept (together with methotrexate and steroids) for four years. She started to complain about physical fatigue, vertigo, disturbance of vision on both eyes (unclear vision), recurrent diplopia, mild difficulty in speech and numbness of the right hand two weeks prior to hospital admission. The neurological examination at the time of admission showed mild paresis of right upper limb and postural tremor of right hand. There was no family history of MS. In 2006 this patient was hospitalized due to dysesthesias of the left limbs, but CT scans were normal. Past medical history was also significant for hypertension and hypercholesterolemia. Routine blood tests were within the normal range, and ANA/ANCA tests were negative. Brain resonance imaging (MRI) showed large hyperintense lesion on T2-weighted images in left hemisphere (frontal, temporal and parietal lobes) without gadolinium enhancement on T1-weighted images (Figure 1A). The CSF analysis revealed normal levels of glucose, mild pleocytosis (18 lymphocytes/mm3), slightly increased protein level (64 mg/dL), intrathecal synthesis of IgG and presence of oligoclonal IgG bands. The treatment with etanercept was discontinued and the patient was treated with intravenous methylprednisolone (1.0g) for 7 days followed by prednisone tapering. On discharge from the hospital, three weeks from initiation of neurological signs, her neurological symptoms had almost completely resolved. At the clinical follow- up visits at 1,2,3,6 months later, the patient was free from neurological symptoms. Follow-up brain MRI showed significant improvement with decreased volume of T2 lesions (Figure1B).
Figure 1: MRI brain showing hyperintense lesions in left hemisphere (frontal, temporal and parietal lobes) (A). MRI brain 3 months later (B).
Discussion
TNF alpha (TNFa), secreted by microglia and macrophages in the CNS, has a direct role in the etiopathogenesis of demyelination and MS [3]. Two forms of TNFa exist: a transmembrane protein (tmTNF) and a soluble form (sTNF), both interacting with two distinct receptors, TNFR1 and TNFR2. Two approaches for specifical targeting the inflammatory effects of TNF include inhibition of sTNF and blocking TNFR1 resulting in reduction of receptor signaling. Results of treatment of different mouse models of RA indicated that TNFR1 signaling is pathologic, whereas TNFR2 signaling might have an anti-inflammatory function [4]. Thus the primary aim in autoimmune diseases is to reduce TNFR1 signaling while preserving interaction with TNFR2 [5]. Accordingly in MS, it has been shown that complete TNF inhibition, including interfering within tmTNF-TNFR2 axis, can cause serious adverse effects [6].
It was previously shown that anti-TNF therapy reduces inflammation in RA but may promote CNS demyelinating disease. It is suggested that anti-TNF agents may promote the risk of demyelinating events even about 30% [7]. Mohan et al in 2001 reviewed cases of neurologic symptoms in patients treated with anti-TNF therapy for RA reported to the U.S. FDA from 1998 to 2000 [8]. Eighteen of a total of 20 patients received etanercept. The most common neurological symptoms were paresthesias, optic neuritis, gait disturbance, weakness and bladder dysfunction. Sixteen of 19 patients who had MR imaging examinations showed lesions compatible with demyelination [7]. In literature there are also descriptions of new-onset MS symptoms in patients with a history of RA treated with etanercept [9,10]. There are also an increasing number of data reporting demyelinating cerebral lesions following anti-TNF treatment detected by MRI imaging without clinical symptoms [11]. Clinical and MRI improvement after discontinuation of anti-TNFa treatment confirm association between inhibition of TNF and CNS demyelination. This correlation is relatively rare but might have serious consequences.
Conclusion
The case described here once again increases our awareness about potential induction of demyelination in patients treated with anti-TNF. The performance of a cranial MRI is strongly recommended in such cases prior to therapy initiation [11]. Close clinical and MRI follow up should help monitor development of demyelination in anti-TNF treated patients.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Robinson WH, Genovese MC, Moreland LW (2001) Demyelinating and neurologic events reported in association with tumor necrosis factor alpha antagonism. Arthritis Rheum 44: 1977-1983.
- Sfikakis PP, Tsokos GC (2011) Towards the next generation of anti-TNF drugs. Clin Immunol 141(3): 231-235.
- Andreadou E, Kemanetzoglou E, Brokalaki Ch, ME Evangelopoulos, C Kilidireas, et al. (2013) Demyelinating Disease following Anti-TNFa Treatment: A Causal or Coincidental Association? Report of Four Cases and Review of the Literature. Case Reports in Neurological Medicine.
- Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G (1999) Impaired on/off regulation of TNF biosynthesis in mice lackig TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10: 387-398.
- Van Hauwermeiren F, Vandenbroucke RE, Libert C (2011) Treatment of TNF mediated diseases by selective inhibition of soluble TNF or TNFR1. Cytokine & Growth Factor Rev 22: 311-319.
- Kassiotis G, Kollias G (2001) Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J Exp Med 193: 427-434.
- Titelbaum D, Degenhardt A, Kinkel RP (2005) Anti-Tumor Necrosis Factor Alpha-Associated Multiple Sclerosis. AJNR Am J Neuroradiol 26: 1548-1550.
- Mohan N, Edwards ET, Cupps TR, PJ Oliverio, G Sandberg, et al. (2001) Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthrides. Arthritis Rheum 44: 2862-2869.
- Sicotte NL, Voskuhl RR (2001) Onset of multiple sclerosis associated with anti-TNF therapy. Neurology 57: 1885-1888.
- Bernatsky S, Renoux C, Siussa S (2010) Demyelinating events in rheumatoid arthritis after drug exposures. Ann Rheum Dis 69: 1691-1693.
- Winkelmann A, Patejdl R, Wagner S, Benecke R, Zettl UK (2008) Cerebral MRI lesions and anti-tumor necrosis factor-alpha therapy. J Neurol 255 [Suppl 6]: 109-114.

 Case Report
Case Report