ABSTRACT
Annona ambotay (Aubl.) (Annonaceae) is known to contain alkaloids, sesquiterpenes, and flavonoids, and antimicrobial activity of its bark, and seeds have been investigated. However, there is limited information available regarding biological activities of its barks. To perform a phytochemical screening of the hydroalcoholic extract from A. ambotay (Aubl.) barks and evaluate different pharmacological activities. The antioxidant activity was performed by the DPPH free radical scavenging method; the antifungal potential was evaluated by the broth microdilution method; cell cytotoxicity by the MTT assay; and lethality assay in Artemia salina. Phytochemical screening revealed the presence of flavonoids, sterols, pentacyclic triterpenes and was also active for annonaceus acetogenins in A. ambotay extract. The results indicated good antioxidant activity with IC50 of 8.30 μg mL-1. Additionally, the antifungal effect of the extract against different strains of Candida sp was observed. About the toxicity in murine fibroblasts (L929), a reduction in cell viability (43% to 84%) was observed; in human keratinocytes (HaCat) there was a reduction in viability (32% to 72%). Cytotoxicity in breast cancer tumor cells evidenced a high antiproliferative effect, with IC50 = 116.32 μg mL-1 (MDA-MB-231), IC50 = 126.87 μg mL-1 (MCF7) and IC50 = 11.04 μg mL-1 (4T1). A toxic effect was evidenced in the Artemia salina assay (LC50 of 296.78 μg mL-1). The extract presented promising biological activities, because of the good antioxidant activity and antiproliferative effect on human cancer cell lines.
Keywords: Annona Ambotay; Antifungal Agent; Antioxidants; Antitumor Agent; Toxicity
Abbreviations: CLSI: Clinical and Laboratory Standards Institute; CFU: Colony Forming Units; DMSO: Dimethyl Sulfoxide; MIC: Minimum Inhibitory Concentration; TSB: Tryptic Soy Broth; MFC: Minimum Fungicidal Concentration; DMEM: Dubelcco’s Modified Eagle’s Medium; SD: Standard Deviation; NCIM: National Collection of Industrial Microorganisms
Introduction
The use of medicinal plants to treat diseases is a common practice among populations worldwide. Due of the great biodiversity that exists, both Brazilian pharmaceutical industry and researchers are interested in native medicinal plants for the development of new therapeutic approaches Dutra, et al. [1]. The Annonaceae family comprises 135 genera and 2,500 species Lúcio, et al. [2], including Annona ambotay. This shrub is distributed throughout South America and is popularly known as envira-cajú or envirataia Maas, et al. [3]. The members of this family provide edible fruits Vendramin, et al. [4] and are used in perfumery, as well as in popular medicine for the treatment of diabetes Madaleno [5] and hypertension Battisti, et al. [6]. Moreover, the genus Annona has different pharmacological properties, such as insecticidal Bravo, et al. [7,8] antitumor Santos Pimenta, et al. [9], antibacterial, cytotoxic Rinaldi, et al. [10], and anticholinesterase activities Formagio, et al. [11]. In popular Bolivian medicine, the seed or bark of A. ambotay is used to treat sprains through direct application to the site of lesion Bravo, et al. [7]. Previously, Takahashi, et al. [12] reported the antibacterial activity of a benzene extract obtained from the bark of A. ambotay against Gram-positive and -negative bacteria. In terms of chemical composition, the presence of alkaloids Leboeuf, et al. [13-15], sesquiterpenes, and flavonoids Bravo, et al. [7] has been demonstrated in extracts from species of the Annonaceae family. From A. ambotay, in addition to the above-mentioned chemical constituents, Oliveira, et al. [14] isolated geovanine and Bravo, et al. [7] isolated argentilactone. The lack of studies on the pharmacological potential of A. ambotay is noticeable. Therefore, this study is the first to perform a screening of potential biological activities of a hydroalcoholic extract of the bark of A. ambotay through of evaluating its antioxidant and antifungal activities, as well as toxic activities against strains of murine fibroblasts, human keratinocytes, human adenocarcinoma mammary gland/breast, murine tumor mammary gland, and Artemia salina.
Materials and Methods
Plant Material
The bark of A. ambotay was purchased at Ervas medicinais (CNPJ 02.117.644/0001-90, Belém, Pará, Brazil). The bark was dried at 40 °C in an oven and then reduced to powder using a knife mill (Metvisa, Brazil).
Preparation of the Extract
Ten grams of dried bark were macerated and extracted with 500 mL of ethanol-water (70:30, v/v) for 72 hours at room temperature. Then, the residue was removed by filtration, and the extract was evaporated to dryness at a lower temperature (<40 °C) under reduced pressure in a rotary evaporator (Buchi, Switzerland), followed by lyophilization (Christ, Germany) under 1.8 mbar pressure and −14 °C. The yield of the extract was 5.9% w/w. The material was stored protected from light at-20 °C until use.
Phytochemical Assay
The phytochemical screening was performed with the dried extract for flavonoids using a 1% aluminum chloride solution in methanol and concentrated hydrochloric acid 36% Kapoor, et al. [16] and for alkaloids using the reactive of Dragendorf Wagner, et al. [17]. The presence of annonaceus acetogenins was achieved by a comparison between results obtained after spraying Dragendorff and Kedde reactives. Samples containing positive spots in both tests were considered active for acetogenins. Tests for sterols and triterpenes were carried out, according to Rizk [18] using Liebermann–Burchard reaction.
Antioxidant Activity
The scavenging activity of A. ambotay bark was measured according to the 1,1-diphenyl-2-picrylhydrazyl free radical (DPPH) method, as described previously by Sreejayan and Rao [19], with minor modifications. Briefly, the sample (50 μL) at different extract concentrations (0.97–250 μg mL−1) was added to each well of a 96- well microplate and mixed with 150 μL of 50 μM DPPH in ethanol solution. The reaction mixture was kept for 30 minutes in the dark at room temperature. Then, the absorbance was measured in a spectrophotometer at 510 nm against the negative control (ethanol). Resveratrol was used as a positive control at the same concentrations. Inhibition of DPPH radical was calculated using Equation 1: IC50 (%) = 100 x (A0 – As) / Ao (Eq. 1), being A0 negative control absorbance and As test-sample absorbance. The IC50 value was calculated from the straight-line equation of the linear dispersion graph and represents the extract concentration that inhibits 50% of DPPH radical. All tests were performed in triplicate.
Antifungal Activity
The standard strains used in this study were as follows: Candida. albicans, American Type Culture Collection (ATCC) 10231; C. glabrata (Taniwaki, M.H.), Collection of Tropical Cultures (CCT) 0728; C. krusei, (FTI) CCT 1517; and C. guilliermondii (CCT) 1890 from the Foundation André Tosello (Campinas, São Paulo, Brazil). The procedures were performed according to the M27-A2 protocol from the Clinical and Laboratory Standards Institute (CLSI) [20]. The fungal suspension was prepared in sterile saline (0.85% NaCl w/v) and then it was diluted in RPMI 1640 culture medium, buffered with 3-(N-morpholino)-propanesulphonic acid (MOPS) and the pH was adjusted to pH 7.0 ± 0.1, to obtain from 5 x 102 to 2.5 x 103 colony forming units (CFU) per mL. The dried extract was diluted in RPMI 1640 medium buffered with MOPS and tween-80/ dimethyl sulfoxide (DMSO) (1:1, v/v). The final DMSO concentration was maintained as less than 1%. Concentrations ranged from 39 to 5,000 μg mL-1 for extract. The assay was performed in 96-well sterile microplates to which 100 μL of analogs dilutions and 100 μL RPMI 1640 were added, buffered with MOPS and inoculated with a suitable number of the microorganism’s colony forming units. The growth control consisted of 100 μL of the same inoculated culture medium and 20 μL mL-1 tween 80/DMSO (1:1, v/v) and a sufficient quantity of the uninoculated medium to make up 200 μL. The negative control was prepared by adding 200 μL of the uninoculated medium. Amphotericin B (Cristália, Brazil) was used as a reference drug at concentrations from 0.0313 to 16.0 μg mL-1. The microplates were incubated at 35 °C for 48 hours. The Minimum Inhibitory Concentration (MIC) was established as the lowest concentration at which no turbidity was observed in the culture medium. After checking the MIC, an aliquot of 20 μL was retained from those wells which showed no visible growth and re-incubated with 4 mL of Tryptic Soy Broth (TSB) without the addition of an antifungal agent, for another 48 hours at 35 °C. The lowest concentration at which no turbidity was noticed after this period was considered to be the Minimum Fungicidal Concentration (MFC).
Cell Viability Assay
The immortalized cell lines [murine fibroblasts (L929), human keratinocytes (HaCaT) human adenocarcinoma mammary gland/ breast (MDA-MB-231 and MCF7) and murine tumor mammary gland (4T1)] were grown in Dubelcco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat-inactivated FBS, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, and 10 mM HEPES and maintained at 37 °C in a 5% CO2 humidified atmosphere at pH 7.2. The cell viability study was performed using the 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay Mosmann [21]. Briefly, all cell types tested were seeded in 96-well plates at a density of 5 × 103 cells in 100 μL of medium per well. After 24 hours of incubation, the culture medium was replaced by fresh medium with the treatments. Quintuplicate wells were treated with A. ambotay extract at concentrations ranging from 7.81 to 1,000 μg mL−1. The plates were incubated at 37 °C in 5% CO2. A control experiment was performed under the same conditions but without cell treatment. After 48 hours, the medium was removed and 90 μL of DMEM with 10 μL of MTT (5 mg mL−1) dye solution was added, followed by incubation for 3 hours at 37 °C. The precipitated formazan was dissolved in DMSO, and the absorbance was measured at 540 nm using a microplate reader. All experiments were performed in a single experiment, and the relative cell viability (%) was expressed as a percentage relative to the untreated control cells. The IC50 value is the concentration of the sample required to inhibit 50% of the cell proliferation and was calculated by plotting the percentage survival vs. the concentrations, using the Microsoft Excel Program.
Brine Shrimp Lethality Assay
The brine shrimp lethality assay was carried out according to Meyer, et al. [22], with some modifications. Encysted eggs of the brine shrimp Artemia salina Leach were obtained from Maramar Aquacultura (Cabo Frio, Rio de Janeiro, Brazil) and incubated in artificial seawater at pH 8–9. After 48 hours of incubation at room temperature, the active nauplii free from eggshells (n=10 units) were collected and added to each set of wells containing dried extract dissolved in 2.5% DMSO and made up to 5 mL total volume using artificial saltwater. The extracts were tested in triplicate at 10 to 1,000 μg mL−1. Thymol and 2.5% DMSO were used as positive and negative controls, respectively (and artificial seawater as negative control too). After 24 hours, the number of survivors was counted, and the percentage of death was calculated. The lethal concentration 50% (LC50 value) and the standard error were calculated by Probit analysis Finney [23].
Statistical Analysis
The results were calculated as a mean ± Standard Deviation (SD). Statistical comparisons were made using the Student t-test, one-way analysis of variance (ANOVA) and Bonferroni’s post-hoc test, using the software PRISM 6 (GraphPad, USA). The limit of statistical significance was set at p < 0.05.
Results and Discussion
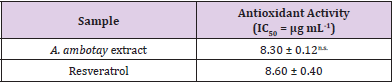
Phytochemical screening revealed the presence of flavonoids, sterols (blue color) and pentacyclic triterpenes (pink color) in A. ambotay extract. The dried extract was active for annonaceus acetogenins (positive both for Kedde and Dragendorf tests). These compounds are in agreement with the typical chemical profile of plants of the Annonaceae family. The antioxidant activity was evaluated for hydroalcoholic extract bark from A. ambotay and the results are depicted in Table 1. The production of oxygen reactive species causes health damages and are involved in the growth of different diseases such as atherosclerosis, rheumatoid arthritis, cancer and neurodegenerative diseases Chen, et al. [24]. Different studies including species of Annona gender describe the antioxidant activity for the extracts of different parts of the plant with the same analytical method used in this study (DPPH). Roesler, et al. [25] found IC50 higher than of this study for the bark extract (IC50= 48.82 μg mL-1) and seeds extract (IC50= 31.14 μg mL-1) from A. crassiflora, as well as Kalidindi, et al. [26] for the chloroform extract of leaves (IC50= 308.3 μg mL-1) from A. squamosa Linn. Moreover, Formagio, et al. [27] found comparable results with this study for the fractions ethyl acetate (IC50 = 8.53 μg mL-1) and hydromethanolic (IC50 = 10.57 μg mL-1) from leaves of A. dioica St. Hill.
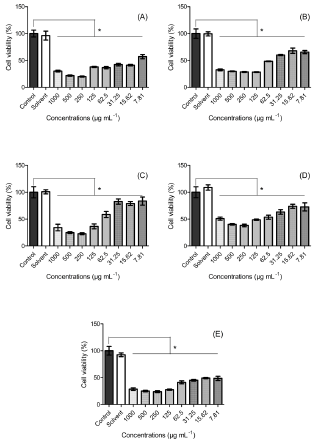
Figure 1:
A. Cell viability of murine fibroblast (L929)
B. Human keratinocyte (HaCat)
C. Breast cancer (MDA-MB-231)
D. (MCF7)
E. And 4T1 with different concentrations of A. ambotay extract.
Note: Data was expressed as mean ± SD (n=5). *p<0.05 compared with control group (one-way ANOVA following Bonferroni post-hoc test).
Table 1: Antioxidant activity of Annona ambotay extract and resveratrol.
Note: The superscript (n.s.) indicates a statistically nonsignificant difference between resveratrol and A. ambotay extract at p < 0.05, as analyzed by Student’s t-test (mean ± SD, n=3).
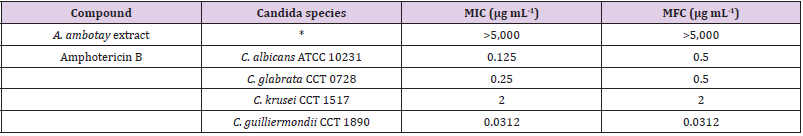
Ruiz-Terán, et al. [28-30] demonstrated a relation between phenolic compounds and antioxidant activity from A. squamosa, A. coriacea, and A. cuneata Oliv, respectively. On the other hand, Lima, et al. [31] isolated twelve acetogenins from A. cornifolia and found an antioxidant activity for this compound with IC50 between 1.95 ± 0.34 μg mL-1 to 0.99 ± 0.18 μg mL-1. Considering the above exposed and the positive phytochemical results for flavonoids and annonaceus acetogenins, it is tempting to suggest that the antioxidant activity of A. ambotay extract can be explained by the presence of these compounds, however, additional experiments are necessary to elucidate this hypothesis. The antifungal activity of A. ambotay bark extract is shown in Table 2. The results show that only reference drug was active against Candida species with MIC value of 0.0312 to 2 μg mL-1, whereas the antimicrobial activity of A. ambotay was >5,000 μg mL-1, which did not demonstrate clinical relevance of the possible use of A. ambotay as an antifungal drug. Padmaja, et al. [32-34] revealed antifungal activity of chemical compounds isolated from Annonaceae species. Okechukwu, et al. [35] analysed the methanol extract leaves from Cleistopholis patens (Annonaceae) and found antifungal potential against clinical strains of Candida albicans (MIC = 9.0 μg mL-1) and Candida krusei (MIC = 9.8 μg mL-1), both isolated from HIV patients in stage II. Additionally, Jamkhande, et al. [36] found the antifungal activity of methanolic extract roots from A. reticulata Linn. against Candida albicans from the National Collection of Industrial Microorganisms (NCIM) 3055. Although of these studies demonstrated antifungal activity of different extracts and different parts of vegetal species from Annonaceae family, in our study, the dried extract of the bark from A. ambotay showed weak antifungal activity, according to the classification described by Kuete [37] who classified plant extracts having MICs of more than 625 μg mL-1 as weak antimicrobial activity.
Table 2: Minimal inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of Annona ambotay extract and reference drug against Candida species.
Note: *for all tested Candida species.
The results of the cell viability assay are reported in Figure 1. It’s shown a reduction of 43% to 84% in cell viability against murine fibroblast (L929) (Figure 1A) shown a cytotoxic effect in all concentrations when compared to the control group. Moreover, in relation to keratinocytes (HaCat), the reduction of cell viability was of 32% to 72% (Figure 1B), when compared to the control group. The cytotoxic effect observed can be associated with the presence of secondary metabolites, among them the acetogenins, important class of compounds present in plants from Annona gender Tundis, et al. [38], whose presence was confirmed in the phytochemical screening. Several activities of acetogenins were reported such as pesticide, antimalarial, antiparasitic and antimicrobial Roham, et al. [39]. Moreover, previously study suggest that the cytotoxicity action mechanism of this compound is related to the capacity to inhibit the complex I of the mitochondrial respiratory chain Bermejo, et al. [40], harming ATP production, necessary to supply energy for cells process. Freiburghaus, et al. [41] analysed the cytotoxicity of ether and dichloromethane extract of bark from A. senegalensis in human fibroblast (WI-38) and concluded that the higher concentration which not influence in the cell growth was 56 μg mL-1 and 6 μg mL-1, respectively. George, et al. [42] evaluated the cytotoxicity of butanolic leaf extract from A. muricata Linn. against human keratinocytes (HaCat) and found IC50 = 30.1 μg mL-1. Comparatively with the studies above, the dried extract from A. ambotay presented higher cytotoxicity effect to murine fibroblast (L929), which had an alteration in the cellular growth with fluctuation of 43% to 84% of cell inhibition in the range concentration tested (7.81 a 1,000 μg mL-1), while the cytotoxicity effect in keratinocytes (HaCat) was smaller (IC50 = 60.65 μg mL-1).
In relation with the cytotoxic effect against breast cancer cell lines, the viability range of 22% to 83% for MDA (Figure 1C), 38% to 74% for MCF7 (Figure 1D) and 23% to 49% for 4T1 (Figure 1E) when compared with the control group. Gavamukulya, et al. [43] analysed the ethanolic extract of leaves from A. muricata in human breast cancer cell (MDA-MB-231) and found dose-dependency with IC50 = 248.77 μg mL-1 for exposition time of 72 hours. Najmuddin, et al. [44] demonstrated the antiproliferative effect of 19 crude extracts of the leaves from A. muricata from different regions of Malaysa and found variation in the IC50 of 221.67 to 799.67 μg mL-1 in breast cancer cell line MCF7 and 350 to 769.44 μg mL-1 in MDA-MB-231 for exposition time of 72 hours. In comparison with the data above, the cytotoxic effect of hydroalcoholic extract of bark from A. ambotay was higher to both cell lines, MDA-MB-231 and MCF7, with IC50 = 116.32 μg mL-1 and IC50 = 126. 87 μg mL-1, respectively. Additionally, the extract showed IC50 = 11.04 μg mL-1 for murine breast cancer cell line 4T1. From the toxicity results of the extract to breast cancer tumor lines, it is possible to observe that the extract may direct its action to the MDA-MB-231 and 4T1 lines, which are characterized by triple negative, that is, presenting lower expression of estrogen receptors, progesterone and human epidermal growth factor receptor 2 (HER2). Holliday & Speirs [45,46] Additionally, the 4T1 cell represents an animal model for stage IV of human breast cancer, exhibiting high metastatic capacity Associação Técnico Científica Paul Ehrlich [47]. Therefore, due to the high toxicity attributed to this cell line, A. ambotay extract represents a possible alternative for the treatment of metastatic breast cancer, usually associated with high mortality.
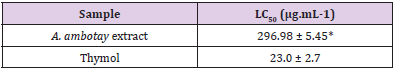
The results of the lethality assay for Artemia salina are described in Table 3. The hydroalcoholic extract from A. ambotay barks showed LC50 of 296.78 μg mL-1. According to Meyer, et al. [22], an extract demonstrates toxicity to A. salina when LC50 <1000 μg mL-1. Therefore, the A. ambotay extract can be classified as toxic. In fact, other extracts obtained from species of this genus have already demonstrated an effect similar to that found. Santos Pimenta, et al. [9] evaluated the toxicity of eighteen different extracts obtained from the seeds, leaves, and trunk of the species A. crassiflora, A. nutans, A. hypoglauca and A. cherimola against Artemia salina and demonstrated their biological activity. The same authors correlated their biological activity with the presence of acetogenins. The ethanolic extracts from leaves and stem bark of A. muricata also showed a toxic effect with LC50 = 324.07 μg mL-1 and LC50 = 196.04 μg mL-1, respectively Silva, et al. [48]. The lethality assay for Artemia salina has shown a good correlation with antitumor activity in vitro, representing an important screening tool for the development of new phytomedicines Arcanjo, et al. [49]. Previous studies on extracts obtained from species belonging to the Annona genus show antitumor action. Suresh, et al. [50] using an ethanolic extract from the roots of A. reticulata, demonstrated significant antiproliferative effect against tumor cell lines: human lung carcinoma (A549), human chronic myelogenous leukemia bone marrow (K-562), human cervix (HeLa) and MDA-MB. Moreover, Chen, et al. [51] using an extract of A. squamosa seeds, evidenced antitumor effect against human tumor cell lines A549 (human lung carcinoma A549 cell line, IC50 3.2 μg mL−1), HeLa (human cervical cancer HeLa cell line, IC50 = 13.0 μg mL−1), MCF-7 (human breast carcinoma MCF-7 cell line, IC50 = 0.25 μg mL−1) and HepG2 (human liver carcinoma HepG2 cell line, IC50 = 0.36 μg mL−1). Taken together, these data associated with the toxic result against Artemia salina, justify the realization of future studies about the antitumor potential of A. ambotay.
Table 3: Lethal concentration 50% (LC50) of the Annona ambotay extract and positive control against the brine shrimp after 24 hours.
Note: The superscript (*) indicates a statistically significant difference between thymol and A. ambotay extract at p < 0.05 as analyzed by Student’s t-test (mean ± SE, n=5).
Conclusion
Altogether, the hydroalcoholic extract bark from A. ambotay demonstrated promising pharmacologic activities such as antioxidant activity and possible antitumoral activity, observed by the effect on viability in breast cancer cell lines and by the experimental protocol of Artemia salina. Furthermore, the results of this study indicate cytotoxicity against cell lines murine fibroblasts (L929) and human keratinocytes (HaCat), associated with discrete antifungal action. Hence, this specie can be used to discover bioactive natural products that may serve as leads in the development of new pharmaceuticals research activities in the future.
Acknowledgement
This work was supported by grants from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Pró-Reitoria de Pós-Graduação e Pesquisa da Universidade Federal de Juiz de Fora (PROPESQ/UFJF), all of Brazil. R.C.D. is recipient of a research productivity fellowship from the CNPq.
Conflict of Interest
There are no conflicts of interest.
References
- Dutra RC, Campos MM, Santos AR, Calixto JB (2016) Medicinal plants in Brazil: Pharmacological studies, drug discovery, challenges and perspectives. Pharmacol Res 112: 4-29.
- Lúcio AS, Almeida JR, Da-Cunha EV, Tavares JF, Barbosa-Filho JM (2015) Alkaloids of the Annonaceae: occurrence and a compilation of their biological activities. Alkaloids Chem Biol 74: 233-409.
- Maas PJ, Maas H, Miralha JM, Junikka L (2007) Flora da reserva ducke, Amazonas, Brasil: Annonaceae. Rodriguésia 58: 617-662.
- Vendramin ME, Costa EV, Santos EP, Pinheiro ML, Barison A, et al. (2013) Chemical constituents from the leaves of Annona rugulosa (Annonaceae). Biochem Syst Ecol 49: 152-155.
- Madaleno MI (2011) Plantas da medicina popular de São Luís, Brasil. Bol Mus Para Emílio Goeldi Cienc Hum 6: 273-286.
- Battisti C, Garlet TMB, Essi L, Horbach RK, Andrade A, et al. (2013) Plantas medicinais utilizadas no município de Palmeira das Missões, RS, Brasil. R Bras Bioci 11: 338-348.
- Bravo JA, Chantraine JM, Saavedra G, Sauvain M (2002) Argentilactone from Annona Ambotay. Rev Bol Quim 19: 6-11.
- Ravaomanarivo LH, Razafindraleva HA, Raharimalala FN, Rasoahantaveloniaina B, Ravelonandro PH, et al. (2014) Efficacy of seed extracts of Annona squamosa and Annona muricata (Annonaceae) for the control of Aedes albopictus and Culex quinquefasciatus (Culicidae). Asian Pac J Trop Biomed 4(10): 798-806.
- Santos Pimenta LP, Pinto GB, Takahashi JA, Silva LG, Boaventura MA (2003) Biological screening of Annonaceous Brazilian Medicinal Plants using Artemia salina (brine shrimp test). Phytomedicine 10: 209-212.
- Rinaldi MV, Díaz IE, Suffredini IB, Moreno PR (2017) Alkaloids and biological activity of beribá (Annona hypoglauca). Rev Bras Farmacogn 27(1): 77-83.
- Formagio AS, Vieira MC, Volobuff CR, Silva MS, Matos AJ, et al. (2015) In vitro biological screening of the anticholinesterase and antiproliferative activities of medicinal plants belonging to Annonaceae. Braz J Med Biol Res 48(4): 308-315.
- Takahashi JA, Pereira CR, Pimenta LP, Boaventura MA, Silva LG (2006) Antibacterial activity of eight Brazilian Annonaceae plants. Nat Prod Res 20(1): 21-26.
- Leboeuf M, Cavé A, Bhaumik PK, Mukherjee B, Mukherjee R (1982) The phytochemistry of the annonaceae. Phytochemistry 21(12): 2783-2813.
- Oliveira AB, Oliveira GG, Carraza F, Maia JG (1987) Geovanine, a new azaanthracene alkaloid from Annona Ambotay Phytochemistry 26(9): 2650-2651.
- Santos PR, Morais AA, Braz-Filho R (2003) Alkaloids from Annona dioica. J Braz Chem Soc 14: 396-400.
- Kapoor LD, Singh A, Kapoor SL, Srivastava SN (1969) Survey of Indian plants for saponins, alkaloids and flavonoids. I Lloydia 32(3): 297-304.
- Wagner H, Bladt S, Zgainski EM (1984) Alkaloid DRUGS. In Plant Drug Analysis. A Thin Layer Chromatography Atlas. Berlin Heidelberg: Springer-Verlag, p. 51-92.
- Rizk AM (1982) Constituents of plants growing in Qatar I. A chemical survey of sixty plants. Fitoterapia 53: 35-39.
- Sreejayan N, Rao MN (1996) Free radical scavenging activity of curcuminoids. Arzneimittelforschung 46(2): 169-172.
- (2002) Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard, document M27-A2, (2nd)., 22.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65: 55-63.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, et al. (1982) Brine Shrimp: A convenient general bioassay for active plant constituents. Planta Med 45(5): 31-34.
- Finney DJ (1971) Probit analysis (3rd)., Cambridge University Press, New York, USA, 60(9): 1432-1432.
- Chen Z, Bertin R, Froldi G (2013) EC50 estimation of antioxidant activity in DPPH· assay using several statistical programs. Food Chem 138(1): 414-420.
- Roesler R, Catharino RR, Malta LG, Eberlin MN, Pastore G (2007) Antioxidant activity of Annona crassiflora: Characterization of major components by electrospray ionization mass spectrometry. Food Chem 104(3): 1048-1054.
- Kalidindi N, Thimmaiah NV, Jagadeesh NV, Nandeep R, Swetha S, et al. (2015) Antifungal and antioxidant activities of organic and aqueous extracts of Annona squamosa leaves. J Food Drug Anal 23(4): 795-802.
- Formagio ASN, Kassuya CAL, Neto FF, Volobuff CRF, Iriguchi EKK, et al. (2013) The flavonoid content and antiproliferative, hypoglycaemic, anti-inflammatory and free radical scavenging activities of Annona dioica St. Hill. BMC Complement Altern Med 13: 14.
- Ruiz-Terán F, Medrano-Martínez A, Navarro-Ocaña A (2008) Antioxidant and free radical scavenging activities of plant extracts used in traditional medicine in Mexico. Afr J Biotechnol 7(12): 1886-1893.
- Benites RSR, Formagio ASN, Argandoña EJS, Volobuff CRF, Trevizan LNF, et al. (2015) Contents of constituents and antioxidant activity of seed and pulp extracts of Annona coriacea and Annona sylvatica. Braz J Biol 75: 685-691.
- Khallouki F, Haubner R, Ulrich CM, Owen RW (2011) Ethnobotanical survey, chemical composition, and antioxidant capacity of methanolic extract of the root bark of Annona cuneata J Med Food 14(11): 1397-1402.
- Lima LARS, Pimenta LPS, Boaventura MAD (2010) Acetogenins from Annona cornifolia and their antioxidant capacity. Food Chem 122(4): 1129-1138.
- Padmaja V, Thankamany V, Hara N, Fujimoto Y, Hisham A (1995) Biological activities of Annona glabra. J Ethnopharmacol 48(1): 21-24.
- Lima LA, Johann S, Cisalpino PS, Pimenta LP, Boaventura MA (2011) In vitro antifungal activity of fatty acid methyl esters of the seeds of Annona cornifoliaSt.-Hil. (Annonaceae) against pathogenic fungus Paracoccidioides brasiliensis. Rev Soc Bras Med Trop 44(6): 777-780.
- Bhattacharya AK, Chand HR, John J, Deshpande MV (2015) Clerodane type diterpene as a novel antifungal agent from Polyalthia longifolia var pendula. Eur J Med Chem 94: 1-7.
- Okechukwu DC, Momoh MA, Esimone CO (2015) Evaluation of the anti-candidal activity of methanolic leaf extract of Cleistopholis patens (fam. Annonaceae) on candida species isolated from stage II HIV patients. Afr Health Sci 15(3): 789-796.
- Jamkhande PG, Wattamwar AS, Pekamwar SS, Chandak PG (2014) Antioxidant, antimicrobial activity and in silico PASS prediction of Annona reticulata root extract. Beni-Suef Univ J Basic Appl Sci, p. 1-9.
- Kuete V (2010) Potential of Cameroonian Plants and Derived Products against Microbial Infections: A Review. Planta Med 76(14): 1479-1491.
- Tundis R, Xiao J, Loizzo MR (2017) Annona species (Annonaceae): a rich source of potential antitumor agents? Ann N Y Acad Sci 1398(1): 30-36.
- Roham PH, Kharat KR, Mungde P, Jadhav MA, Makhija SJ (2016) Induction of mitochondria mediated apoptosis in human breast cancer cells (T-47D) by Annona reticulata leaves methanolic extracts. Nutr Cancer 68(2): 305-311.
- Bermejo A, Figadere B, Zafra-Polo MC, Barrachina I, Estornell E, et al. (2005) Acetogenins from Annonaceae: recent progress in isolation, synthesis and mechanisms of action. Nat Prod Rep 22(2): 269-303.
- Freiburghaus F, Kaminsky R, Nkunya MH, Brun R (1996) Evaluation of African medicinal plants for their in vitro trypanocidal activity. J Ethnopharmacol 55(1): 1-11.
- George VC, Kumar DR, Rajkumar V, Suresh PK, Kumar RA (2012) Quantitative assessment of the relative antineoplastic potential of the n-butanolic leaf extract of Annona muricata in normal and immortalized human cell lines. Asian Pacific J Cancer Prev 13(2): 699-704.
- Gavamukulya Y, Abou-Elella F, Wamunyokoli F, AEl-Shemy H (2014) Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac J Trop Med Supp 7: S355-S363.
- Najmuddin SUFS, Romli MF, Hamid M, Alitheen NB, Rahman NMANA (2016) Anti-cancer effect of Annona Muricata Linn Leaves Crude Extract (AMCE) on breast cancer cell line. BMC Complement. Altern Med 16(1): 311.
- Holliday DL, Speirs V (2011) Choosing the right cell line for breast cancer research. Breast Cancer Res 13(4): 215.
- Abu N, Zamberi NR, Yeap SK, Nordin N, Mohamad NE, et al. (2018) Subchronic toxicity, immunoregulation and anti-breast tumor effect of Nordamnacantal, an anthraquinone extracted from the stems of Morinda citrifolia L. BMC Complement Altern Med 18(1): 31.
- (2018) Associação Técnico Científica Paul Ehrlich. 4T1 cell line.
- Silva EMF, Nascimento RBC, Barreto FS, Filho MOM, Griz SAS, et al. (2015) Estudo in vitro do potencial citotóxico da Annona muricata Rev Ciênc Farm Básica Apl 36: 277-283.
- Arcanjo DDR, Albuquerque ACM, Melo-Neto B, Santana LCLR, Medeiros MGF, et al. (2012) Bioactivity evaluation against Artemia salina Leach of medicinal plants used in Brazilian Northeastern folk medicine. Braz J Biol 72(3): 505-509.
- Suresh HM, Shivakumar B, Hemalatha K, Heroor SS, Hugar DS, et al. (2011) In vitro antiproliferative activity of Annona reticulata roots on human cancer cell lines. Pharmacognosy Res 3(1): 9-12.
- Chen Y, Xu SS, Chen JW, Wang Y, Xu HQ, et al. (2012) Anti-tumor activity of Annona squamosa seeds extract containing annonaceous acetogenin compounds. J Ethnopharmacol 142(2): 462-466.

 Research Article
Research Article