Abstract
Development of suitable analytical method is important for a specific measurement. At the same time the method has to be validated to confirm the suitability for the intended use. The quality, consistency and reliability of the test result can be judged by the typical method validation characteristics through repeatability, detection limit, quantitation limit, working range in terms of linearity, trueness, bias, recovery etc. Liquid chromatographic technique has been used in the current study to estimate the soluble sulphur content in rubber chemical sulphur by the use of less quantity chemical having comparatively less health hazards.
Keywords:Method Development; Working Range; Repeatability; Detection Limit; Quantification Limit
Abbreviations:ISO: International Organization for Standardization; ASTM: American Society for Testing and Materials; IS: Indian Standard; NBS: National Bureau of Standards; CNS: Central Nervous System; HPLC: High Performance Liquid Chromatography
Introduction
An analytical method is a process or pathway to estimate,
determine, or solve any type of measurand or problem or
product [1,2]. It needs some instruments, tools, glassware,
mathematical theorems to solve the problem [3]. Various national
and international bodies, e.g. International Organization for
Standardization (ISO), European Committee for Standardization
(CEN), American Society for Testing and Materials (ASTM), Indian
Standard (IS), National Bureau of Standards (NBS) are involved in
this work to create standard analytical methods with respect to
quality and reliability [4,5]. In rubber industry various standard
methods are used for testing purpose such as characterization
of incoming raw materials as well as in process materials, and
finished products [6]. Such a method is ASTM D4578: “Standard
Test Method for Rubber Chemicals – Determination of Percent
Sulfur by Extraction [7].” At rubber product manufacturing units,
Sulfur is used as curing or vulcanizing or cross-linking agent [8,9].
Two allotropes of Sulfur are used: soluble Sulfur (S8 crown) and
insoluble Sulfur (polymeric) [10]. For better performance of rubber
product, insoluble Sulfur is preferred during rubber compounding
[11,12]. Use of soluble Sulfur in rubber matrix leads to blooming
phenomena and also makes the rubber product more prone to
oxygen and ozone attacks [11,13].
It is essential to quantify the fraction of soluble Sulfur present in
the total Sulfur to be used for curing purpose [14]. The S8 allotrope
of Sulfur is soluble in Carbon disulfide (CS2) and Toluene (C6H5CH3)
[15]. In the ASTM D4578 method, one of these two solvents is
used for extraction purpose, leaving behind the insoluble material.
Total amount of solvent required for extraction is 100 to 150
ml over a minimum extraction period of 8 minutes [7]. CS2 is a
colourless, highly volatile, obnoxiously odorous, flammable, toxic
liquid solvent having both acute and chronic toxicity. Exposure
of CS2 having concentration of 10 ppm or more in atmosphere
may lead to numbness, blurred vision, cramps, muscle fatigue,
neurophysiological impairment, psychosis, keratitis, priapism,
erectile dysfunction, and death by respiratory failure [16]. On
the other hand for acute (short-term) and chronic (long-term)
exposure of toluene toxicity, affect Central Nervous System (CNS)
primarily in both humans and animals. Acutely exposed to elevated
airborne levels of toluene; symptoms include fatigue, sleepiness,
headaches, and nausea [17].
We have developed liquid chromatographic [High Performance
Liquid Chromatography (HPLC)] technique as a successful
analytical method for the estimation of soluble sulphur content in
total sulfur. Here also we have used toluene but very less quantity
(5 ml) is sufficient to get repeatable and reliable result. Necessary
study has been done to establish as a successful validated method
[18].
Material and Method
Materials
HPLC grade toluene and methanol were procured from Merck India. Double distilled deionised water was prepared in Millipore water system, Elga, USA. Soluble sulphur (CAS No.: 7704-34-9, Product No. 84683) having purity more than 99.5% has been procured from Sigma Aldrich. Waters glass vials were used for sample and standard solution preparation. Borosil micropipette and volumetric flask were used for volume measurement and standard solution preparation. Ministar syringe filter was used for sample solution filtration. All chemicals were used without further treatment and purification.
Instrument
Liquid chromatography model: Alliance HPLC System, Waters
e2695 separations module with 2998 PDA detector was procured
from Waters India Pvt Ltd, Bangalore. The instrument parameters
were set as:
a. Column C18: pore size 100 Å, particle size 5 μm, inner diameter
4.6 mm and length 250 mm
b. Column temp: 40°C
c. Injection volume: 10μl
d. Sampling type: Auto sampler
e. Solvent A: 100% Methanol
f. Solvent B: 100 % water
g. Total flow rate: 1.3 ml/min
h. Flow detail: 98% Pump A + 2% Pump B (Isocratic)
i. Detector Wavelength: 285 nm
Method
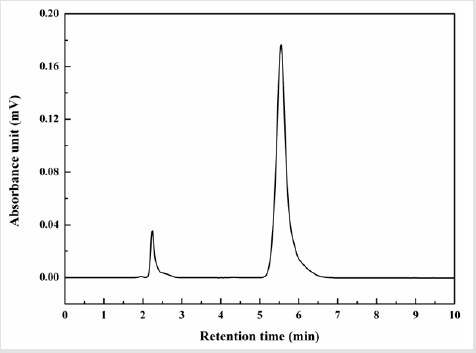
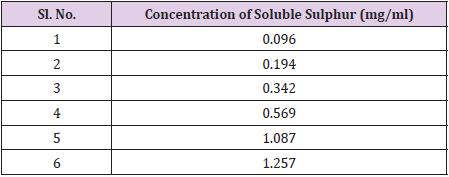
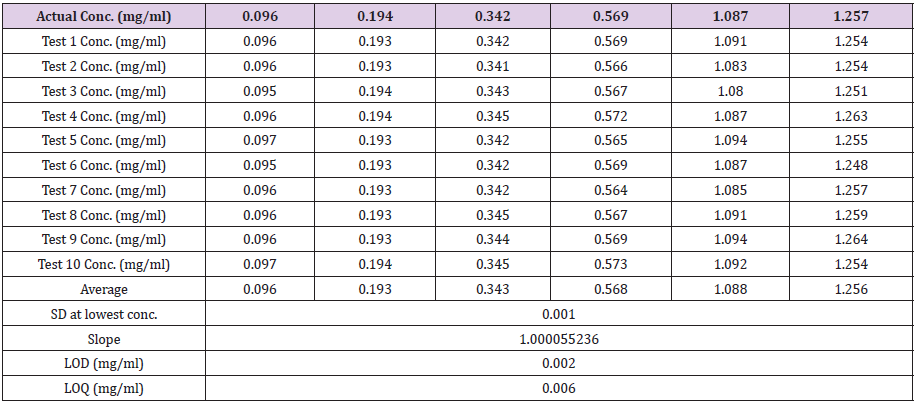
A set of calibration standards (Table 1) solution have been prepared by dissolving pure soluble sulphur in 5 ml HPLC grade toluene. The solution has been filtered through syringe filter and injected in HPLC using the above program (Section 2.2). A typical HPLC chromatogram has been shown in Figure 1. First peak at ~2.3 min represents elution of toluene and the second peak at ~5.6 min was for soluble sulphur. The area under the second peak was epitomized to soluble sulphur was taken with respect to the concentration of the sulphur separately for each prepared calibration solutions. A calibration curve between area under the curve and concentration of soluble sulphur was drawn. From the obtained linear equation, soluble sulphur content in test specimen has been estimated.
Result and Discussion
Repeatability Measurement
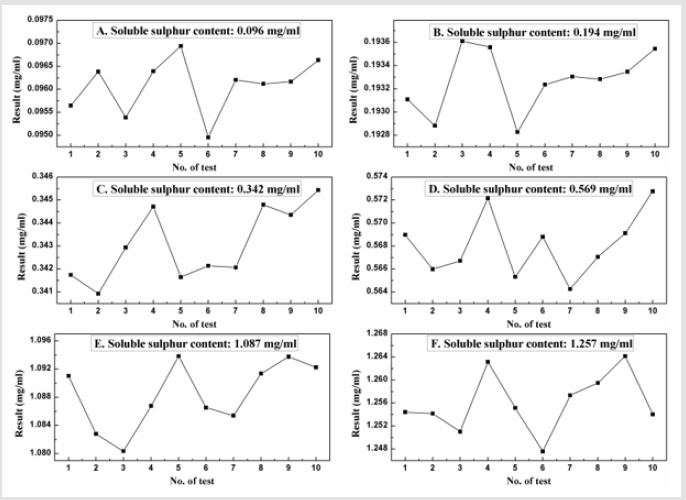
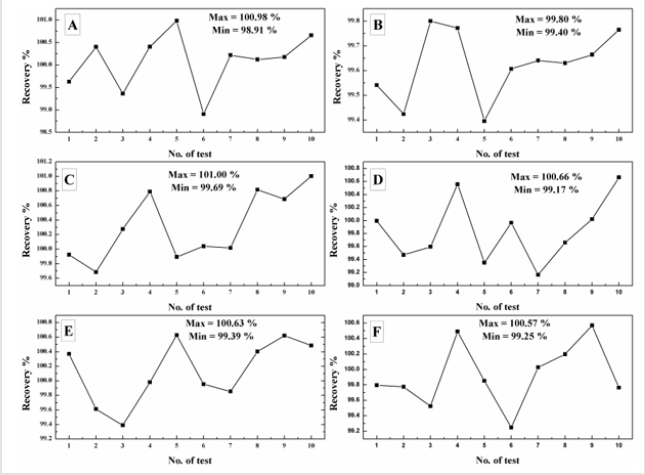
It is the experiment performed under a set of similar condition to evaluate how repeatable the results are. Ten replicate tests of each calibration standard samples have been analysed to study the repeatability of developed technique and results are tabulated in Table 2 and all results have been plotted separately in Figures 2A- 2F. The closeness of the results clearly indicates the repeatable data obtained in all cases.
Limit of Detection (LOD) and Limit of Quantification (LOQ)
Limit of detection is defined as the lowest concentration of an analyte in a sample that can be detected, though not necessarily quantitated. Limit of quantitation is defined as the lowest concentration of an analyte in a sample that can be determined with acceptable precision and accuracy understated operational condition of the method. LOQ is directly proportional to the precision. LOD (Eq. 1) and LOQ (Eq. 2) are calculated from the standard deviation (Eq. 3) at lowest concentration using the following equations [19].
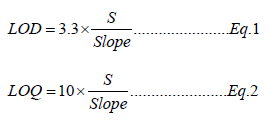
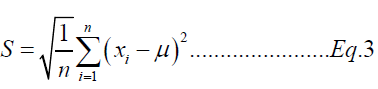
Where,
S: Standard deviation of ten replicates data of lowest calibration standard sample (Table 2)
xi: ith test result of lowest calibration standard sample
μ: Mean of ten replicates data of lowest calibration standard sample
n: Number of replicate test performed (n = 10)
Estimation of Working Range
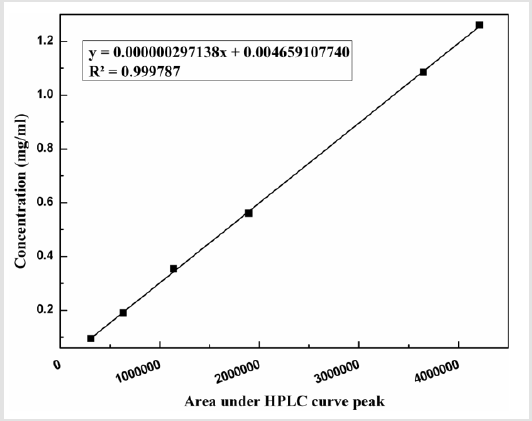
Working range of a method defines the interval over which the uncertainty will be acceptable. To estimate the working range, linearity of response obtained from different calibration standards tested and achieved correlation coefficient as 0.9998. The linearity study has been shown in Figure 3.
Trueness
Trueness is defined as closeness of a single value to the mean
of infinite number of result which practically determination is not
possible. In place of mean of infinite data i.e. true value, actual metal
content in the calibration standard has been considered. Trueness
of this method has estimated in term of bias and precision.
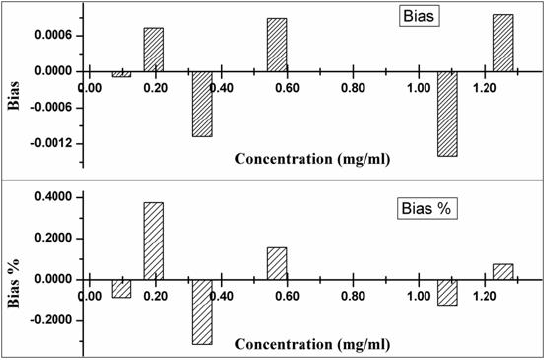
Bias is defined as the closeness of the result with true value
and can be estimated by three different techniques, (a) analysis
of reference material (b) recovery of the analyte (c) comparison
of results obtained from different techniques. In this study, the
calibration standards have been considered as reference material,
bias, percent bias and recovery have been estimated and results are
mentioned in Figures 4 & 5.
Conclusion
While estimating soluble sulphur content using HPLC as a tools, it was found that regression coefficient factor obtained during calibration is 0.9998 which signifies that the developed method is exhibit linear relationship during analysis of standard sample having concentration from 0.096 mg/ml to 1.257 mg/ml. Trueness study of standard samples indicates both the positive and negative bias obtained during the analysis and the maximum is 0.376% and -0.315% respectively. Recovery in different concentration has been analysed and found it is lying between 98.91%-101.00%. The experiment of estimation of limit of detection and limit of quantification confirms that, through this technique, soluble sulphur having concentration 0.002 mg/ml can be detected in the sample and can be quantified above 0.006 mg/ml. Thus the above technique can be adopted as an analytical method as far as environment point of view with respect health hazardous effect and less quantity chemical use.
Acknowledgement
The authors wish to acknowledge ISO/IEC 17025:2017 accredited Analytical and Chemical Department, HASETRI and JK Tyre & Industries for providing the research facility and financial support to undertake the research work.
References
- Khusna AH (2020) Analytical thinking process of student in proving mathematical argument. Int J Sci Technol Res 9(1): 1248-1251.
- Suits LD (2007) National and international standards governing geosynthetics. In: RW Sarsby (Eds.)., Geosynth. Civ Eng. Woodhead Publishing, UK, p. 66-93.
- Harvey D (2019) Basic Tools of Analytical Chemistry.
- I Standards. Appendix-International Standards Part B Measurement Methods for Composition and Structure, (n.d.), pp. 1097-1149.
- Report S (2019) Rubber standards in today’s world economy. Rubber Plast News, p. 14-18.
- Aiza Jaafar CN, Zainol I, Ishak NS, Ilyas RA, Sapuan SM (2021) Effects of the liquid natural rubber (LNR) on mechanical properties and microstructure of epoxy/silica/kenaf hybrid composite for potential automotive applications. J Mater Res Technol 12: 1026-1038.
- (2017) ASTM D4578-06. Standard Test Methods for Rubber Chemicals-Determination of Percent Sulfur by Extraction, ASTM Int (n.d.).
- Kruželák J, Kvasničáková A, Hložeková K, Vilčáková J, Hudec I (2021) Influence of Combined Sulfur and Peroxide Curing Systems and Ageing on the Properties of Rubber Magnets. Macromol Symp 395: 2000258.
- Vieyres A, Pérez-Aparicio R, Albouy PA, Sanseau O, Saalwächter K, et al. (2013) Sulfur-Cured Natural Rubber Elastomer Networks: Correlating Cross-Link Density, Chain Orientation, and Mechanical Response by Combined Techniques. Macromolecules 46(3): 889-899.
- Meyer B (1976) Elemental sulfur. Chem Rev 76(3): 367-388.
- Rodgers B, Waddell WH, Klingensmith W (2004) Rubber Compounding. Encycl Polym Sci Technol. American Cancer Society.
- Shahrampour H (2018) Comparison of Sulfur Curing Systems (Insoluble-Rhombic) on Physical and Thermal Properties of the Matrix Polymeric of Styrene-Butadiene Rubber and Natural Rubber. Pet Chem 58: 721-726.
- Datta RN, Huntink NM, Datta S, Talma AG (2007) Rubber Vulcanizates Degradation and Stabilization. Rubber Chem Technol 80(3): 436-480.
- Wrȩczycki J, Bieliński DM, Anyszka R (2018) Sulfur/organic copolymers as curing agents for rubber. Polymers (Basel) 10(8): 870.
- Lens P, Pol LWH (2000) Environmental Technologies to Treat Sulfur Pollution. IWA Publishing.
- (2021) Carbon disulfide. Wikipedia. Free Encycl (n.d.).
- Yavari F, Van Thriel C, Nitsche MA, Kuo MF (2018) Effect of acute exposure to toluene on cortical excitability, neuroplasticity, and motor learning in healthy humans. Arch Toxicol 92(10): 3149-3162.
- (2012) National Association of Testing Authorities. Guidelines for the validation and verification of quantitative and qualitative test methods. Nata, p. 1-32.
- Shrivastava A, Gupta V (2011) Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles Young Sci 2(1): 21-25.

 Research Article
Research Article