Abstract
Background: Rapid and accurate identification of patients with Coronavirus disease-2019 (COVID-19) is necessary for applying an adequate management strategy to reduce morbidity and mortality. Molecular and immunological diagnosis of COVID-19 requires suitable laboratory infrastructure. This work aims to study the use of inflammatory markers for the diagnosis of COVID-19.
Methods: This case-control study included patients with reverse transcriptionpolymerase chain reaction (RT-PCR)-confirmed COVID-19 and healthy participants. Patients were recruited from the National Hepatology and tropical research institute. Routine laboratory markers, Ferritin, D-Dimer, Pro-calcitonin, C-reactive proteins (CRP), Tumor necrosis factor-alpha (TNF-Alpha), interleukin-6 (IL-6) levels were assessed. Regression analysis was performed to detect the independent markers associated with COVID-19 diagnosis and receiver operating characteristic (ROC) curves were used.
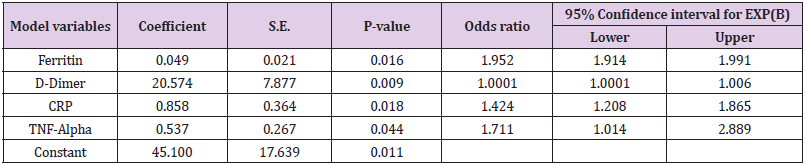
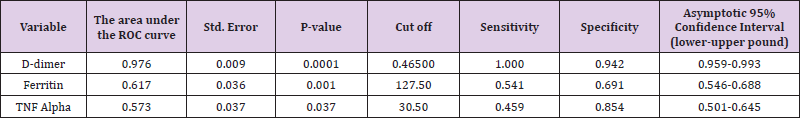
Results: This study included 135 patients with PCR-confirmed COVID-19 and 137 healthy controls. Patients with COVID-19 were significantly older with no difference among the 2 groups regarding their gender distribution or body mass index. Logistic Regression revealed that Ferritin (OR:1.952, 95%CI: 1.914- 1.991, P-value:0.016), CRP (OR:1.424, 95%CI:1.208-1.865, P-value:0.018), D-Dimer (OR:1.0001, 95%CI:1.0001- 1.006, P-value:0.009), TNF-Alpha (OR: 1.711, 95%CI: 1.014-2.889, P-value: 0.044), were the independent variables associated with the diagnosis of COVID-19. D-dimer at cut off 0.465 has sensitivity: 100% and specificity:94.2%, AUC:0.976. Ferritin at cut off 127.50 has sensitivity 54.1% and specificity 69.1%, TNF-alpha at cut off 30.50 has sensitivity:45.9% and specificity:85.4%
Conclusion: Ferritin, D-Dimer, CRP, and TNF-Alpha can be used in directing the diagnosis towards COVID-19 infection particularly during the pandemic till COVID-19 specific PCR is available.
Keywords: COVID-19; Ferritin; D-Dimer; Tumor necrosis factor-alpha; diagnosis; Egypt
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARSCoV- 2), causing coronavirus disease 2019 (COVID-19), emerged as a multifaceted problem in December 2019. The first case infected with the newly discovered SARSCoV-2 in Africa was reported in Egypt on February 14, 2020 [1]. Egypt reached the first peak of SARS-CoV-2 infections in June-July 2020 [2]. Diagnosis of SARS-CoV-2 infection is based on immunological and molecular tests. Immunological tests include the detection of viral-specific antibodies in serum (diagnose previous infection, infection of at least 3-4 weeks’ duration, asymptomatic infections in close contacts) or SARS-CoV-2 antigens in samples from respiratory secretions (diagnose current infection) [3-5]. Molecular testing is the nucleic acid amplification test (NAAT) including real-time polymerase chain reaction (RT-PCR) to diagnose current infection. The performance of RT-PCR depends on the type and quality of the test and the respiratory secretions specimen in addition to the duration of COVID-19 disease. False-negative results ranged from <5 to 40% [3-5]. The favored initial diagnostic test is RTPCR. In some settings, antigen detection may be used initially but with lower sensitivity. A negative antigen detection testing should be confirmed with NAAT [6]. Molecular tests require adequate laboratory infrastructure [5].
If possible, all symptomatic patients with suspected SARACoV- 2 infection should be tested. However, in some poor areas, limited capacity could preclude testing all suspected individuals. Local health authorities may have specific criteria for testing. Different non-specific laboratory biomarkers were used to support the diagnosis, prognosis, and monitoring of patients infected with COVID-19. Although nonspecific, some biomarkers were reported to be linked to the infectious process of SARS-CoV-2. Previous studies and meta-analyses found that the most common laboratory abnormalities reported with COVID-19 have decreased serum albumin, platelet count, lymphopenia, elevated CRP, lactate dehydrogenase, transaminases, and ESR [7,8] as well as increased D-dimer and low hemoglobin [9].
Other abnormalities reported were increases in neutropenia, total bilirubin, creatinine, cardiac troponin, PT, and PCT [10,11]. In some countries, biomarkers such as CRP, PCT, lymphopenia, and IL-6 and IL-10 are already being used to aid diagnosis or to provide early evidence of more severe disease progression [12]. cytokine storms with high levels of IL-2R, IL-6, IL-10, and TNF-α and a reduction in the absolute numbers of CD4+ and CD8+ T lymphocytes have been related to severe COVID-19, with progression to cardiovascular collapse, multiple organ failure, and rapid deaths [5]. This work aimed to study simple laboratory markers that could be involved in the diagnosis of early mild COVID-19 disease within a cohort of Egyptian patients.
Materials and Methods
This case-control study included consecutive patients with and without COVID-19 disease presented at the National Hepatology and Tropical medicine Research Institute (NHTMRI) who agreed to participate in the study and provide blood samples.
Ethics Statement
All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study was approved by the ethical committee of the General Organization for Teaching Hospitals and Institutions (GOTHI), Number ITH0031. Written informed consent was obtained from all patients before they participated in the study.
Subjects
This study included adults above 18 years, male and female genders. Patients with a confirmed diagnosis of SARS-CoV-2 virus infection (defined as a positive result of real-time reversetranscriptase polymerase-chain-reaction (RT-PCR) assay for nasal and pharyngeal swab specimens) were recruited. Written informed consent was obtained from all patients before they participated in the study. We included patients with mild COVID-19 disease at hospital admission and whose symptoms started within one week before admission. The severity of COVID-19 was determined according to the management protocol of the Egyptian Ministry of Health and Population. Mild cases are defined as symptomatic cases with lymphopenia or leukopenia with no radiological lung affection by pneumonia [13].
Routine laboratory investigations were done including complete blood picture (CBC), Erythrocyte sedimentation rate (ESR), Liver function tests: (serum Bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyltransferase (GGT), alkaline phosphatase (ALP), Prothrombin time, Prothrombin concentration), Kidney function tests: (blood urea nitrogen, serum creatinine), lactate dehydrogenase (LDH), and D-dimer. Inflammation biomarkers: (Serum ferritin (determined using the chemiluminescence immunoassay (CLIA)), C-reactive protein (CRP), Pro-calcitonin (measured by Chemiluminescence), Tumor necrosis factor-alpha (TNF-Alpha) (measured by TNF-Alpha ELISA Assay Kit, Immunodiagnostic, Germany, normal value is <20 pg/ml), Interleukin-6 IIL-6 (Roche Cobas e411 (Roche Diagnostics GmbH, Mannheim, Germany. The upper limit of normal is 7 pg/ml)) were assessed according to manufacture instructions. Regression analysis was performed to detect the independent markers involved in the COVID-19 diagnosis.
Statistical Analysis
Data collection and cleaning were done using Microsoft excel 365©, while for Statistical analysis IBM SPSS 26 (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp) was used. Descriptive statistics were done to explore, summarize and present the data. For hypothesis testing, we used Mann-Whitney tests and Chi-square tests with a significance level of 5%. Multivariate analysis was also done fitting a logistic regression model using Enter method. Furthermore, a receiver operating characteristic curve was established as a form of classification model to examine the threshold of D dimer in the diagnosis of COVID-19 infection.
Result
This case-control study included 135 patients with PCRconfirmed non-severe COVID-19 disease and 137 healthy controls. We recruited the participants during the period from1st of May to the 15th of July, 2020. There was no difference among the 2 groups regarding their age, gender distribution, comorbidities, or BMI. All Patients with COVID-19 disease had mild to moderate disease and all recovered and were discharged from the hospital (Table 1). Patients with COVID-19 disease showed significantly higher white blood cell count, neutrophils, lymphocytes, and monocytes percentages. Inflammatory markers of Serum ferritin, Pro-calcitonin, LDH, and CRP were significantly higher in patients with COVID-19 disease. D-dimer, Tumor necrosis factor-alpha, and Interleukin-6 serum levels were significantly higher in patients with COVID-19 disease (Table 1).
Multiple logistic regression analysis was performed to assess the impact of several factors on the likelihood that patients would have a COVID-19 infection. The model contained 7 independent variables (age, ferritin, D-dimer, TNF alpha, procalcitonin, CRP, and IL-6). The full model containing all predictors was statistically significant, X2 350.5, P-value <0.0001, indicating that the model was able to distinguish between cases that had or did not have COVID-19. The model explained between 72.8% (Cox and Snell R square) and 97.1% (NagelKerke R squared) of the variance in COVID-19 infection and correctly classified 98.5% of cases. As shown in Tables 2&3 most of the independent variables included in the model made a unique statistically significant contribution to the model at a significant level of 5%, and if we considered the significant level of 1% all the independent variables will be significant. The strongest predictor of having a COVID-19 was TNF alpha, recording an odds ratio of 1.7. D-dimer at cut off 0.465 has sensitivity: 100% and specificity: 94.2%, AUC: 0.976. Ferritin at a cut off 127.50 has a sensitivity of 54.1% and specificity of 69.1%, TNF-alpha at a cut-off of 30.50 has sensitivity: 45.9% and specificity: 85.4%.
Table 3: Cut off values and Area under the ROC curve (AUC) of each marker for the diagnosis of COVID-19.
Discussion
The number of patients infected with COVID-19 is currently increasing all over the world in both developed and developing countries. Evaluation and management of the disease depend on its severity. Most cases are mild and recover within 14-21 days. In some poor areas, limited capacity could preclude the availability of molecular testing of all suspected cases. Local health authorities may have specific criteria for testing and the management may depend on the prevalence of COVID-19 in the area or history of travel to high-risk regions [14,15]. Another situation that raises the importance of auxiliary laboratory studies is negative nucleic acid amplification testing in suspicious symptomatic patients [16,17]. In such a situation, the clinical diagnosis of COVID-19 could be supported by distinctive laboratory or imaging results which can be a reason to establish infection control measures [18]. In addition to their diagnostic role, initial cytokines assessments are predictable of disease outcome and, accordingly, arouse the importance of utilizing serum cytokine levels for treatment decisions [19].
This study aimed to use routine laboratory and inflammatory markers to help in the diagnosis of mild COVID-19 cases that can be managed by home isolation preserving molecular diagnosis in low-income countries for severe cases to optimize and prioritize hospitalization. We chose these markers because they were proved to be elevated during the disease course of COVID-19. They also were proved to affect disease severity and mortality. This study included 135 patients with PCR-confirmed non-severe COVID-19 disease and 137 healthy controls. Multiple Logistic Regression revealed that Ferritin, D-Dimer, CRP, TNF-Alpha, were the independent variables for the diagnosis of COVID-19. The inflammatory response to the SARS-CoV-2 virus is reflected in the laboratory results of infected patients according to the severity of their disease [15]. Wang et al, 2020 found that D-dimer, CRP, ESR, and lactate dehydrogenase were significantly lower in patients with mild disease than in severe cases while procalcitonin did not show any increase in mild cases [15].
In our study, serum ferritin was within the normal range but significantly higher in COVID-19 patients. Ferritin is a protein that is iron storing. It increases during viral infection and reflects active viral replication [20]. Patients with mild COVID-19 disease usually present with normal ferritin levels on hospital admission (30–400 μg/L) [14]. Elevated serum ferritin levels (>400 μg/L) were usually observed in patients with severe disease on admission [21]. A meta-analysis by Cheng and colleagues, 2020 that involved 10 614 COVID-19-confirmed cases, reported that ferritin level is significantly higher in patients with severe COVID-19, nonsurvivors, patients with one or more chronic diseases [20].
TNF-alpha starts to rise early in COVID-19 infection and continues to rise during the disease [21]. Chen and colleagues, 2020 [10] found that IL-2R, IL-6, and TNF-α increase with the increasing severity of COVID-19. Although IL-6 levels were significantly higher in univariate analysis in patients with COVID-19 of our study, it was not proved by multivariate analysis to help in the diagnosis of mild COVID-19 cases. IL-6 was proved to be a trustworthy indicator of disease severity and outcome of mechanical ventilation [22]. A study by Del Valle et al. 2020 [19] that included confirmed COVID-19 cases of different severities (from mild to severe cases with organ damage) found that IL-6 followed by TNF-alpha were the highly independent factors associated with severity and prediction of outcome. They used a cut-off above 70 pg ml−1 for IL-6 while in our study the mean value of IL-6 in COVID-19 patients was 28.5±9.8 pg ml−1. They used a cut-off above 35 pg ml−1 for TNF-α which was near our mean value of TNF- α of 32.5±12.8 pg ml−1. They found that IL-6 levels were the most dynamic, but they did not compare their levels among patients with different COVID-19 severities.
Disturbed coagulation profile and overt disseminated intravascular coagulation is an early indicator of high mortality in patients with COVID-19. D-dimer appears in the blood after coagulation occurs. D-dimer was reported to be a strong independent factor of mortality in COVID-19 [14]. During the early stages of COVID-19 disease, D-dimer level is usually normal or slightly increased [23] like our study. Limitations of the current study include the small sample size, and only a few markers are assessed and other cytokines which could have an important role were not measured.
Conclusion
This work showed that ferritin, D-Dimer, CRP, TNF-Alpha, were the independent laboratory variables associated with the diagnosis of Egyptian patients with mild COVID-19 who did not progress to severe disease, recovered and discharged. Serum Ferritin, D-Dimer, CRP, and TNF-Alpha can be used in directing the diagnosis towards COVID-19 infection and can be a reason to establish infection control measures, particularly during the pandemic till COVID-19 specific-PCR is available.
Funding Statement
This research received no specific grant from any funding agency, commercial or not-for-profit sectors. The data that support the findings of this study are available on request from the corresponding author, [Shousha HI].
Disclosure of Interest
The authors report no conflicts of interest.
References
- Medhat MA, El Kassas M (2020) COVID-19 in Egypt: Uncovered figures or a different situation? J Glob Health 10(1).
- (2020) Worldometers.info. Dover, Delaware, U.S.A.
- Cheng MP, Papenburg J, Desjardins M (2020) Diagnostic Testing for Severe Acute Respiratory Syndrome-Related Coronavirus 2: A Narrative Review. Ann Intern Med 172: 726.
- Weissleder R, Lee H, Ko J, Pittet MJ (2020) COVID-19 Diagnostics in Context. Sci Transl Med 12(546).
- Pizzol JLD, Hora VPD, Reis AJ, Jilia V, Ivy R, et al. (2020) Laboratory diagnosis for Covid-19: A mini-review. Rev Soc Bras Med Trop 53.
- Patel A, Jernigan DB, 2019-nCoV CDC Response Team (2020) Initial Public Health Response and Interim Clinical Guidance for the 2019 Novel Coronavirus Outbreak - United States, MMWR Morb Mortal Wkly Rep 69(5): 140-146.
- Rodriguez-Morales AJ, Cardona-Ospinaa JA, Gutiérrez-Ocampoa E, RV Pena, YH Rivera, et al. (2020) Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis.
- Yang X, Yang Q, Wang Y (2020) Thrombocytopenia and Its Association with Mortality in Patients with COVID-19. J Thromb Haemost.
- Lippi G, Plebani M (2020) Laboratory abnormalities in patients with COVID-2019 infection Clin Chem Lab Med.
- Chen G, Wu D, Guo W, Y Cao, Da H, et al. (2020) Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130(5): 2620-2629.
- Lippi G, Plebani M (2020) The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin Chem Lab Med 58(7).
- Green K, Allen AJ, Sukla J, FR Beyer, DA Price, et al. (2020) What is the role of imaging and biomarkers within the current testing strategy for the diagnosis of Covid-19? The Centre for Evidence-Based Medicine at the University of Oxford.
- (2020) Ministry of Health and Population, Egypt Management protocol for COVID-19 Patients.
- Velavan TP, Meyer CG (2020) Mild versus severe COVID-19: Laboratory markers. Int J Infect Dis 95: 304-307.
- Wang F, Hou H, Luo Y, G Tang, S Wu, et al. (2020) The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight 5(10).
- (2020) Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19.
- (2020) World Health Organization. Diagnostic testing for SARS-CoV-2. Interim guidance.
- Caliendo A, Hanson K (2020) Coronavirus disease 2019 (COVID-19): Outpatient evaluation and management in adults. Upto Date.
- Del Valle DM, Kim-Schulze S, Huang HH (2020) An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 26(10): 1636-1643.
- Cheng L, Li H, Li L, C Xu, S Yan, et al. (2020) Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J Clin Lab Anal 34(10).
- Gómez-Pastora J, Weigand M, Kim J (2020) Hyperferritinemia in critically ill COVID-19 patients - Is ferritin the product of inflammation or a pathogenic mediator? Clin Chim Acta 509: 249-251.
- Fara A, Mitrev Z, Rosalia RA, Assas BM (2020) Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol 10.
- Grobler C, Maphumulo SC, Grobbelaar LM (2020) Covid-19: The Rollercoaster of Fibrin (Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes. Int J Mol Sci 21(14).

 Research Article
Research Article