ABSTRACT
A silver-colored heavy metal Chromium (Cr) which exhibits different oxidation states (-2 to +6) has physical properties like being lustrous, brittle, odourless and, tasteless. It has many physicochemical and biochemical properties due to its trivalent chromium (Cr(III)) and chromium (Cr(VI)) electronic structure. Being an extensive metal it has many industrial uses such as in electroplating, leather tanning, pigments, petroleum refining etc. It is a major concern to us as it is non-biodegradable in nature, its chemical treatment results in production of more secondary pollutants. Consequently, when the heavy metals are emitted out of the industries into the environment their concentration should be brought down to an admissible level. The chemical remediation of the Cr(VI) residues is expensive. Hence, bioremediation of this toxic Cr(VI) metal is the focus of this article. Microorganisms or microcosms that can remediate Cr(VI) exhibit distinctive strategies (depends upon the quality, bio-sorbent and further estimating the presence of reductants) such as biosorption, biotransformation and bioreduction. It is very beneficial for Cr(VI) removal in this way, overcoming all the difficulties related between research facilities and modern applications for chromium remediation.
Keywords: Hexavalent Chromium; Trivalent Chromium; Bioreduction; Biosorption; Biotransformation; Bioaccumulation; Toxicity; Chromate Reductase
Introduction
Chromium was first discovered by Louis Nicholas Vauquelin as an element (PbCrO4) along with molybdenum and tungsten that belong to the transition group (VI-B) in the modern periodic table [1-5]. This heavy metal is toxic to environment as it contaminates the soils and sediments along with surface and groundwater due to man-made activities. ATSDR (Agency for Toxic Substances and Diseases Registry) states that Cr(VI) is listed under the 17 most toxic chemicals. Chromium occur in several states but the two most stable states are: one being soluble and highly toxic Cr(VI) and the other being comparatively less soluble and toxic Cr(III), which is 10-100 times less bio-available and more stable. Cr(VI), most toxic form of chromium as it is a strong clastogen because it has high oxidation potential, greater solubility in water and further can easily penetrate through biological surfaces. Cr(VI) before being ejected out from the tanneries into the external environment needs to be treated properly before outflow in the environment. Cr(VI) also has an adverse impact on plants such as like alteration in seed germination, reduced growth of roots, stems and leaves. Waste like phenol, chloride, sulphide, tannins and formaldehyde are generated by tanning of Chromium. Even, less toxic Cr(III) gets converted into highly toxic Cr(VI) upon oxidation during this process. Chromium sulphate [Cr2(SO4)3] is used in treating leather. As Cr(VI) is a very stable compound it can sometimes get deposited in the food chain which leads to diseases like stomach irritation, cancer in digestive tract, brain damage, diarrhea, premature death in mammals etc. that can even be even fatal to higher organisms. According to USEPA (United States Environmental Protection Agency) hexavalent chromium is regarded as priority pollutant and listed as class ‘A’ human carcinogen [6-10].
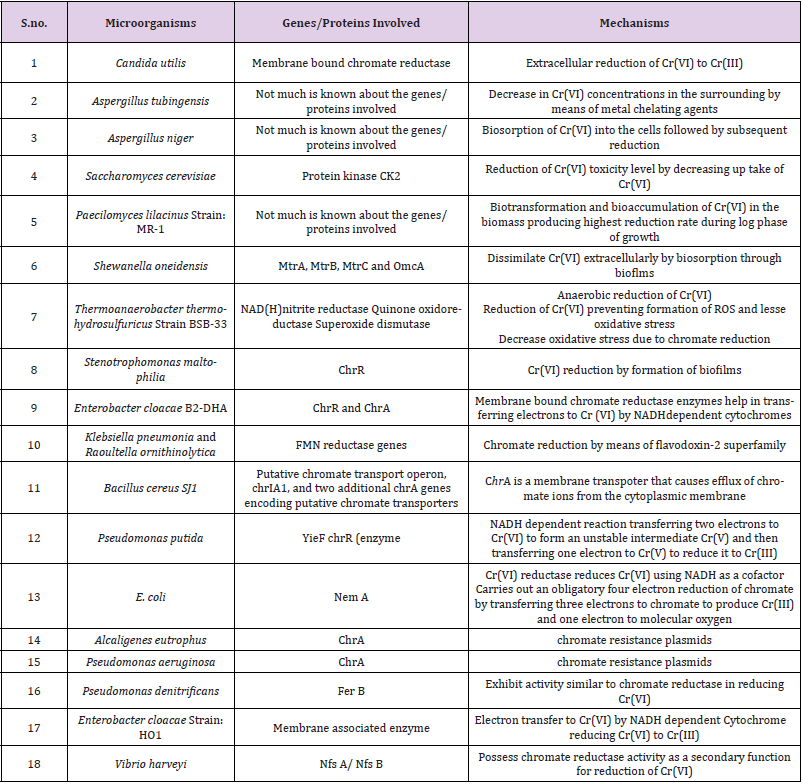
Table 2: Microorganism’s genes involved in Cr(VI) biodegradation along with their molecular mechanism.
Some of the methods like reverse osmosis, precipitation , chemical reduction followed by ion exchange and absorption (coal, activated carbon and alum) are generally used to purify the Cr(VI) residues released from the industrial effluents. Besides, being economically expensive the major drawback of these methods is partial removal of metal, high reagent and requires too much energy which leads to the production of toxic material which again need disposal. Most practical methods for reducing Cr(VI) which is toxic in nature to less toxic form Cr(III) is by biological reduction of Cr(VI) through microorganisms and their enzymes [11,12]. Cr(VI) can also be reduced through wide variety of bacteria which are, Staphylococcus aureus, Stenotrophomonas maltophilia, Pediococcus pentosaceus etc., in presence of air(aerobic) or absence of air(anaerobic) or both. The ability of bacteria to reduce is due to environmental conditions and further, Cr resistant bacteria can undergo bioreduction for long duration. Structural similarity among the molecules, leads to Cr(VI) intake through the sulfate transport channel which occurs in bacterial membranes due to rapid mutation in contaminated environment. Chromium reducing bacteria has different chromate reductase genes such as ChrR, YieF, LpDH and NemA, that are used in catalysing depletion of Cr(VI) to Cr(III). Major source of Chromium contamination is through leather industry, concentration (Between 2000 to 5000 mg/L) which is much more from the acceptable range (2 mg/L) in case of effluents. The aim of this article is to confess the dangerous impact of Cr(VI) including strategies for the use of either naturally occurring or deliberately introduced microorganisms to consume and break down Cr(VI) pollutant in order to remediate the polluted site (bioremediation) [13-15].
Essentiality of Trivalent Chromium
Cr can occur in several oxidation states, depending upon the human exposure. Cr(III) is a part of GTF(Glucose Tolerance Factor), which has an important dietary component that increases the action of insulin and hence regulate the carbohydrate metabolism. Insulin activity is also enhanced by Cr(III), when it attaches to insulin, further boosts its impact by triple times. Thus, Cr deficiency may cause diseases related with Carbohydrates and weight loss [16]. Even nutritional supplements, foods and multivitamins consist of Cr(III) carrying compounds. The supplementation products are as follows: CrPic (Chromium Picolinate), CrHis (Chromium histadinate), CRDC (Chromium dinicocysteinate), and NBC (Niacin-bound Chromium). As Cr(III) compounds cannot cross the membranes, so they get collected around the cells which leads to the variations in functioning of cells and damage of membrane in cell. It was reported that Cr(III) containing healthy addition for example CrPic can encourage DNA damage outcomes. Within biological media, series of chemical changes can also undergo in Cr (III). In 2016, Cr(III) was the fourth most selling supplement in USA. Behind calcium, magnesium, and iron one can take Cr(III) in their diet within the range of 23-29 μg/day .The dietary intake of Cr(III) has been beneficial for diabetes and in the process of anabolism of muscle and loss of weight according to some studies. But the evidence of it is not very strong and pretty controversial, moreover some studies even revealed that too much intake of chromium is carcinogenic [17-22].
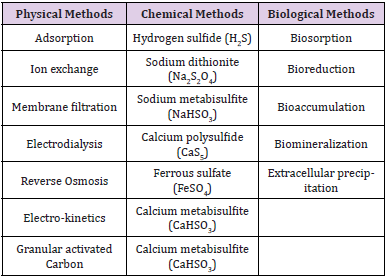
Physical and Chemical Methods for Remediation of Cr(VI)
Physio-chemical properties of substances for remediation is explained through physical and chemical methods. Physical methods of remediation involves techniques like electrodialysis, adsorption, membrane filtration, capping, photocatalysis, soil washing etc. whereas chemical remediation involves the use of varied variety of chemicals like sodium metabisulfite, sulphur dioxide, sodium sulfite, ferrous sulphate barium sulfite, limestone and lime are used for reducing Cr(VI) to Cr(III). Photocatalysis is a process through which Cr(VI) can be reduced to Cr(III). Cr(VI) reduction can be done through organic acids that are conjugated with the absorption of Cr(III) in soil. Cr(VI) reduction to Cr(III) is highly impacted depending upon soil type, its texture, composition, pH, conductivity, and many different physical properties. However, all the transition metal ions which are found in soil can acts as catalyst during the process of reduction. Manganese found in soil materials plays a key role in Cr(VI) reduction rapidly to Cr(III) [23- 26].
The photolytic Cr(VI) reduction through Titanium dioxide (TiO2) at pH 3 even when the organic compounds are present or absent depending upon the recent studies. Cr(VI) ions in water can also be reduced through highly active photocatalyst which is Lanthanum titanium oxide (La2Ti2O7). Few of the physical and chemical methods are feasible and help metal recuperation, whereas some of the procedures are not that effective and cannot be used daily as it is an expensive procedure to carry out, requires pretty high energy consumption and cannot synthesis the secondary pollutants. Most of these procedures work efficiently when there is a presence of high concentration of metal and its effectiveness depends upon the amount agents with which it can interfere. For the promotion of sustainable and economic method for heavy metal removal the disadvantages of physical and chemical methods are needed to be considered. For typical cleanup technologies bioremediation is a good alternative. Bioremediation is the effective procedure which can eliminate heavy metal pollutants from the living bodies. For metal bioremediation the usage of microbial techniques, comprises of great ability to eliminate heavy metals depends upon its less cost and less secondary waste generating technique. Hence, bioremediation is seen to be an effective methodology for eliminating Cr from polluted environment in an eco-friendly and economical manner (Table 1).
Molecular Mechanisms involved in Bioremediation of Chromium
Different research workers discovered the molecular mechanism to reduce the toxic Cr(VI) to Cr(III) by biosorption, bioaccumulation and bioreduction. Bacterial cells on coming in contact with increased concentration of chromium starts producing ROS(Reactive Oxygen Species) to tolerate Chromium stress. Different bacteria have different mechanism to tolerate the high Cr concentration. Chromium can attach to the cell surface of the microorganisms by a chemical bond which is formed between the functional groups that are found on the cell surface structures of microorganisms which has a different glycolipid, proteins, glycoproteins, polysaccharides, and metal ions. Cr(VI) gets reduced into Cr(III) and gets precipitated on the surface of the membrane tissue during adsorption [27-30]. The reduction is catalyzed by a chromate reductase gene or occurs spontaneously.
Through sulfate transport channel present on the membranes of the eukaryotes and prokaryotes the chromate gets transported very actively. With the help of certain specific metal binding proteins, chromium gets transported and finally it gets reduced into less toxic Cr(III), via highly unstable oxidative states. When oxygen is present, any endogenous reserve or NADH, NADPH serve as electron donor but when oxygen is absent Chromium(VI) itself serves as the terminal electron acceptor on the respiratory chain and it often utilizes proteins from cytochrome families like cytochrome b and cytochrome C Efflux of the excess Chromium(VI) from microbial cells usually occurs through a transmembrane protein (efflux pump) and it is encoded by plasmid gene ChrA. Same type of methods has been developed in plants to protect them against Chromium, such as the accumulation of Chromium ions in vacuoles, their binding with organic ligands, scavenging of Chromium generated ROS via antioxidative enzymes (Table 2).
Bioremediation using Bacteria
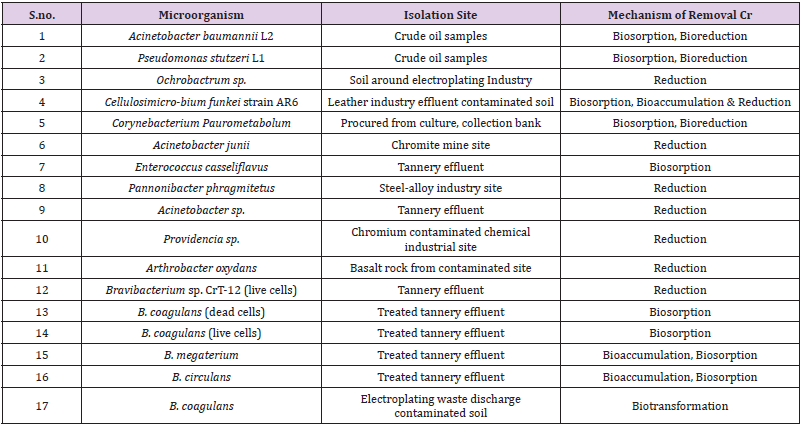
Bacterial remediation in case of Chromium is counted as an easy , economic, and little energy consuming procedure which can be easily accomplished by utilizing native, not harmful strains of bacteria. The transformation of Hexavalent chromium into less toxic Trivalent chromium is usually done by utilizing the microbes. Evacuation of Cr(VI) by bioreduction, biosorption and bioaccumulation could be easily done by microorganisms (both gram positive and gramnegative). The misuse of electroplating of industry diminished Cr(VI) by utilizing a solvent chemical and malate as an outer electron contributor, Bacillus coagulans can be isolated. Bacillus circulans, Bacillus megaterium and Bacillus coagulans are known for its interesting capacity of chromium biosorption. Moreover, Bacillus coagulans and Bacillus megaterium exhibit living and dead cells capacity for biosorption which were contradicted, and dead cells were found to be more powerful for chromium biosorption. For example, Bacillus coagulans living cells absorbed 23.8mg Chromium g-1 dry weight, whereas dead cells absorbed 39.9 mg Chromium g-1 dry weight. The comparable outcomes for B. megaterium were found 15.7 mg Chromium g-1 for living cells whereas 30.7 mg Chromium g-1 dry weight for dead cells. But the better presentation of latent/dead biomass was observed for living cells because of their defenceless action for poisonous impacts of metal particles which can prompt cell passing during the metal expulsion measure [31].
In some of the experiments performed with intact cells and cell free extracts of Arthrobacter species and Bacillus species, it was found that Bacillus species could be substandard when compared with Arthrobacter species as far as their resistance to hexavalent chromium and its decreasing capacity. Reduction of Cr(VI) due to the cell free extract of Arthrobacter species, demonstrated the inclusion of certain solvent proteins in the reduction process. It has been more broadly elucidated the contribution of a dissolvable protein during reduction of Cr(VI) to Cr(III) by Pseudomonas putida and this protein can use NADH or NADPH as an electron donor. How Pseudomonas species can evacuate the cancer-causing Cr(VI) by the usage of a blended populace has also been reported. This report got much more highlight due to the decrease of chromate through a sulfur lessening bacterium which was detached from metal sullied marine residue of Tokwanan, Hong Kong in absence of air. There was huge decrease of chromate brought in in 168hours by the improvement of bacterial consortium (98.5%).
This microbial consortia were developed by immobilized cells of Pseudomonas aeruginosa, Bacillus subtilis and Saccharomyces cerevisiae on a strong help which developed a huge ability in expulsion of Cr(VI) from tannery effluent. The microbial consortium which was created by using native microscopic organisms from tannery squander defiled biotopes can lessen Cr(VI) at different focuses going from 100 to 300 mg Chromium L-1 of a wide scope of pH, utilizing an assortment of electron contributors. Initially Pseudomonas species was found to obstruct towards Cr(VI), later on, this mechanism was found in Cupriavidus metallidurans, Ochrobactrum tritici and so on Chromium safe microorganisms with high remediation productivity under both high impact and in absence of air have been dissociated from highly polluted premises of TCCL(Tamil Nadu Chromates and Chemicals Limited), 0.15mg of bacterial cells g-1 of soil and molasses have been used to fill greatest chromate decrease as a carbon source. Gram positive bacterium Corynebacterium paurometabolum which has properties of biosorption, just as biotransformation, also contributes to limit the poisonousness of chromium mixes can also be utilized (Table 3).
Fungi Being used in Bioremediation
Fungi has also been reported and introduced for remediating the sites which are contaminated by Chromium. In case of fungi biosorption has been found to be productive than bioreduction. In Aspergillus oryzae, Aspergillus niger, Trichoderma species and Fusarium oxysporum the biosorption of Chromium has been discovered. Both metabolism dependent and independent approaches are utilized by fungi for Chromium biosorption. During adsorption of Chromium(VI) on the fungal cell surface it usually forms a chemical bond with the functional groups which are usually present on the cell surface proteins. The system which helps to depict the functional groups Carboxyl, carbonyl and hydroxyl helps to bind the Cr(VI) on the fungal cell wall surface. With the help of fungal strains many biosorptive products had been evolved and they have been studied how to remove chromium in an effective way. Carica papaya plant dry stems were used for the development of a biosorbent matrix in which cells of Fusarium oxyporum were collected. At the 5th day of incubation, the highest level of Chromium biosorption was achieved (90%). When Aspergillus flavus biomaterial was charged with Fe2+ ions, it promotes the removal Chromium and the stickiness involved with the biomaterial which was developed. Even strains of marine seaweed like Aspergillus flavus and Aspergillus niger were tested for its capability of its capacity and accumulation of Cr(VI). The results were found to be more than 25% of the supplied Cr(VI). Some fungi like Hypocrea tawa and Trichoderma inhamatum can reduce Cr(VI) to Cr(III) [32].
According to some studies even yeast can reduce to Cr(VI). Two yeast strains i.e., Pichia anomala M10 and Pichia jadinii M9 are isolated from the effluent of textile dry industry by them. Yeast with the help of chromate reductase (ChrR) enzyme can help to convert Cr(VI) into Cr(III), which is less toxic. According to some investigations biosorption of Cr(VI) is possible from aqueous solution and even sometimes from sewage of tannery industry with the help of Biomass /Polymer Matrices Beads (BPMB). By using immobilizing baker’s yeast in alginate extract(3%) these beads were designed and Chromium concentration was reduced under favourable conditions at 200ppm of initial Chromium concentration. Even administration of fungal bacterial biofilms can remove toxic Chromium(VI). From the contaminated sites of Chromium: Aspergillus, Fusarium and Penicillium, are usually isolated. Aspergillus has the tolerance at 200g/mL, 600g/mL, 650g/mL with 5000g/mL for total chromium. Other fungus like Aspergillus flavus have had the tolerance of Chromium(VI) 600g/ mL with 800g/mL for total chromium, Aspergillus niger has 600g/ mL and 1000g/mL for total Chromium and Avicularia versicolor has the tolerance of 1000g/mL for Chromium(VI).
Conclusion
Uncontrollable and uneven excretion of heavy metals contaminated industrial effluent into the environment has led to a major concern for the environment of 21st century. Some techniques like biosorption and bioreduction has become effective for the remediation of highly toxic and carcinogenic Cr(VI). Biomass of microorganisms, fungi, plants, algae has been reported effective for the compelling of highly toxic hexavalent chromium reduction. The molecular remediation system also gave a better vision about Cr(VI) removal. These all helped in building up the current advances of chromium remediation to be progressively proficient. There is a wide gap between lab discoveries and efficient usage of bioremediation modification for elimination of Cr(VI). Many, important trials are practiced involving artificial solutions of chromium to investigate proficiency of the made framework of remediation in case of chromium. The ability in case of natural frameworks for the cure of Hexavalent Chromium polluted soil and mechanical effluent can be found. Moreover the descriptive discoveries are from the cluster method of the treatment with small quantity of effluent. If we all are looking into all these issues, then the invention of integrated method for the better and continuous elimination of Hexavalent chromium from industries are not much far enough.
Till now we all are working on the culture dependent techniques which involves the culturing of organisms in the artificial laboratory conditions but this technique is not totally reliable for the remediation of toxic heavy metals as we know that very low number of microbes can be grown or cultured in artificial media in laboratories so we need to switch our work towards the culture independent techniques which involves metagenomics etc so that we can work in a broader way and on more microorganisms. By using culture independent technique basically we can explore more on an environmental community of microorganisms and not only on a single organism. This technique may reduce the gap between the laboratory work and its application on the remediation of Cr(VI) on the actual site in environment.
References
- Kanojia R, Junaid M, Murthy R (1998) Embryo and fetotoxicity of hexavalent chromium: a long-term study. Toxicol Lett 95(3): 165-172.
- Arslan P, Beltrame M, Tomasi A (1987) Intracellular chromium reduction. Biochim. Biophys. Acta Mol Cell Res 931(1): 10-15.
- Roundhill D, Koch H (2002) Methods and techniques for the selective extraction and recovery of oxoanions. Chem Soc Rev 31(1): 60-67.
- Kamaludeen SP, Megharaj M, Juhasz AL, Sethunathan N, Naidu R (2003) Chromium-microorganism interactions in soils: remediation implications. Rev Environ Contam Toxicol 178: 93-164.
- Bielicka A, Bojanowska I, Wisniewski A (2005) Two faces of chromium pollutant and bioelement. Pol J Environ Stud 14(1): 5-10.
- Pechova A, Pavlata L (2007) Chromium as an essential nutrient: a review. Vet Med 52(1): 1-18.
- Browning CL, Wise JP (2017) Prolonged exposure to particulate chromate inhibits RAD51 nuclear import mediator proteins. Toxicol Appl Pharmacol 331: 101-107.
- Medeiros MG, Rodrigues AS, Batoreu MC, Laires A, Rueff J (2003) Elevated levels of DNA-protein crosslinks and micronuclei in peripheral lymphocytes of tannery workers exposed to trivalent chromium. Mutagenesis 18(1): 19-24.
- Shahid M, Shamshad S, Rafiq M, Khalid S, Bibib I, et al. (2017) Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere 178: 513-533.
- Volesky B (1990) Biosorption and biosorbents. In: Volesky B (Edt.)., Biosorption of Heavy Metals. CRC Press Florida, USA, p. 3-82.
- Kornhauser C, Wrobel K, Wrobel K (2002) Possible adverse effect of chromium in occupational exposure of tannery workers. Ind Health 40(2): 207-213.
- Lu Z, Ouyang X, Zhang W, Lu X (2013) Isolation of Cr(VI) resistant bacteria and exploration of Cr(VI) removal mechanism of strain N-9. Appl Mech Mater 295-298: 74-77.
- Shiller AM, Boyle EA (1987) Variability of dissolved trace metals in the Mississippi River. Geochem Cosmochim Acta 51(12): 3273-3277.
- Xingzhen Q, Xiuxia L (1987) Investigation on the natural background values and states of elements in natural water from the upper reaches of the Nenjiang river. Kexue Tongbao 32(14): 983-987.
- (1987) US Environmental Protection Agency. Office of Drinking Water. Health advisory chromium, Washington, DC, USA.
- Meranger JC, Subramanian KS, Chalifoux C (1979) A national survey of cadmium, chromium, copper, lead, zinc, calcium and magnesium in Canadian drinking water supplies. Environ Sci Technol 13: 707.
- Fonds AW, Van den Eshof AJ, Smit E (1987) Water Quality in the Netherlands. National Institute of Public Health and Environmental Protection, Bilthoven, Netherlands. Report no. 218108004.
- Papp JF (2004) Chromium use by market in the United States. 10th International Ferroalloys Congress. Proc: Marshalltown, South Africa, The South African Institute of Mining and Metallurgy, Cape Town, South Africa, pp. 770-778.
- Megharaj M, Avudainayagam S, Naidu R (2003) Toxicity of hexavalent chromiu and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr Microbiol 47(1): 51-54.
- Park D, Yun YS, Jo JH, Park JM (2000) Biosorption process for treatment of electroplating wastewater containing Cr (VI): laboratory-scale feasibility test. Ind Eng Chem Res 45: 5059-5065.
- Thacker U, Datta M (2006) Reduction of toxic chromium and partial localization of chromium reductase activity in bacterial isolate DM1. World J Microbiol Biotechnol 21: 891-899.
- Chandra P, Sinha S, Rai UN (1997) Bioremediation of Cr. from water and soil by vascular aquatic plants. In: ACS Symposium Series 664: 274-282.
- (2010) Tamil Nadu Pollution Control Board. Revised Action Plan for Critically Polluted Area e Ranipet.
- Jobby R, Desai N (2017) Bioremediation of heavy metals. In: Kumar P, Gurjar BR, Govil JN (Eds.)., Biodegradation and Bioremediation. Environmental Science and Engineering. Studium Press, New Delhi, India, pp. 201-220.
- Wang L, Wang N, Zhu L, Yu H, Tang H (2008) Photocatalytic reduction of Cr(VI) over different TiO2 photocatalysts and the effects of dissolved organic species. J Hazard Mater 152(1): 93-99.
- Jacobs J, Hardison RL, Rouse JV (2001) In- situ remediation of heavy metals using sulfur- based treatment technologies. Hydrovisions 10: 1-4.
- Barrera Díaz C, Lugo V, Bilyeu B (2012) A Review of Chemical, Electrochemical and Biological Methods for Aqueous Cr(VI) Reduction. 223-24: 1-12.
- Zouboulis AI, Loukidou MX, Matis KA (2004) Biosorption of toxic metals from aqueous solutions by bacterial strain isolated from metal-polluted soils. Process Biochem 39(8): 909-916.
- Rani A, Kumar A, Goel R (2008) Bioremediation a natural approach for heavy metal contaminated site. In: Saikia R (Eds.)., Microbial Biotechnology. New India Publishing, India, pp. 207-227.
- Asha Latha P, Sandeep Reddy S (2013) Review on bioremediation potential tool for removing environmental pollution. Int J Basic Appl Chem Sci 3(3): 21-33.
- Adhikari T, Manna MC, Singh MV, Wanjari RH (2004) Bioremediation measure to minimize heavy metals accumulation in soils and crops irrigated with city effluent. J Food Agric Environ 2(1): 266-270.
- Garbisu C, Alkorta I (2003) Basic concepts on heavy metal soil bioremediation. Eur J Miner Process Environ Protect 3: 58-66.

 Review Article
Review Article