Abstract
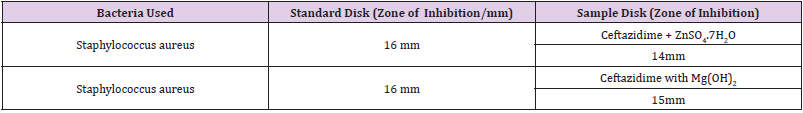
The Ceftazidime is an antibiotic of third-Generation of Cephalosporin. The cephalosporins have appeared as one of most widely ordered classes of the antibiotics in United States. The tremendous escalation of group of drug has been accompanied by the great perplexity as to their suitable use. Improper exercise of the cephalosporins has consequence in the bacterial resistance, excessive costs and clinical failures. The ceftazidime has been also used successfully in the Lower Respiratory Tract Infections, Skin and Skin-Structure Infections, Urinary Tract Infections, Bone and also Joint Infections, Central Nervous System Infections and also Intra-abdominal Infections. Ceftazidime has been interacted with Zn, Mg as the in-vitro examination. More to the point, the anti-microbial analysis of drugs along with complexes was determined. It has also scrutinized that the Ceftazidime interrelates with the metal and also antacid on pH 7.4 by the plotting a variety of UV Spectrophotometric methods. This examine also authenticates that there was a interaction between Ceftazidime with metal and also antacid which was the authenticated by the job’s plot technique additionally by the antimicrobial exploration. This was also authenticated that the region of the inhibition of Ceftazidime with Metal as well as antacid squeezed from 16 mm to 14 mm and 15 mm correspondingly. The typical Ceftazidime disk checked against Staphylococcus aureus.
Keywords: Ceftazidime; Spectrophotometry; Antimicrobial Action; Complexation; Job’s Plot
Introduction
Ceftazidime is the semisynthetic, beta-lactam, broadspectrum, antibacterial drug for the parenteral administration. This is a pentahydrate of pyridinium compound [1]. At this time drug interaction be competent of the simply be dissimilar as the interaction connecting a drug in addition to additional material with intention of the stop medicine commencing the phase as expected [2]. These correlations may happen by reason of be short of the knowledge regarding the principle ingredients associated in related substances [3]. Two medicines are antagonistic at what time their interaction occurs a reduce in special effects of one or else both of medicines. The dissimilar responses of receptor to act of a medicine has effected in number of arrangements, which use expressions such as “partial agonist”, “competitive agonist” etc. This is even expected that a lot of authors would mismanage any given arrangement [4] Commonly iron complexes were used in convey of oxygen in human blood as well as tissues. An mature at rest takes 250ml of uncontaminated oxygen for each minute, these oxygen conceded by metal complex convey system recognized heame, alloying oxygen to go away the blood at what time it arrives the tissue [5]. Antibiotic and metal interaction and successfully found there after interaction result. Also, the antimicrobial activity of drug and the metals complexes were determined. This has been seen that the antibiotic interacts along with metal at the pH 7.4. The different essential metal intricate of the many drugs has been synthesized in addition to characterized by the techniques like NMR, UV, atomic absorption, FT-IR as well as elemental analysis. Spectroscopic, IR Spectroscopic, disk diffusion method, Biological assay studies of complexes [6]. On the other hand in this interaction there uses metals complex which are interaction with drug into our body. And have been use there various type of bacteria for finding zone of inhibition. Some paper there uses methanol and ethanol for disk diffusion result clearly showing [7].
Materials & Methods
(Tables 1 & 2) Ceftazidime stock solutions 250 milliliter of the 1x10-2 Molar was set by the softening 1.386 gm of Ceftazidime solution in 250 milliliters of Demineralized Water (DMW) in a 250 milliliters volumetric flask. The reserve solutions were thinned to the desired potency by buffer solutions [8].
Preparation of Metal Solutions
For the basis of 0.01 Molar Zn solution such as zinc sulfate hepta hydrate ( precisely 0.28754 gm) was assessed exactly in addition to the initiated with assist of funnel in the 100 ml volumetric flasks, softened in the DM water in addition to the framework to mark by the alike solvent. These principal solutions were supplementary diluted ten folds in equivalent solvent in company with the concluding solution was 0.0001 M concentration.
Preparation of Antacid Solutions
For the grounding of 0.01 Molar antacid solution like Mg(OH)2 (0.0740gm) was assessed accurately as well as initiated with facilitate of the funnel in the 100 milliliters volumetric flask, and softened in demineralized water also construct up to the mark by identical solvent. This primary solution was further diluted ten crinkles in the identical solvents and the final also solutions were 0.0001 M concentrations.
Grounding of the Buffer Solutions
To get set buffer solution 1.76 grams of disodium hydrogen phosphate was softened in demineralized water by 2.43 grams of solution dihydrogen phosphate in addition to the pH was adjusted to pH 7.4 as well as volume was also completed to 1000 milliliters with equivalent solution.
Grounding of the Typical Curve of the Ceftazidime
Ceftazidime reserve solutions at pH 7.4 in addition to the concentrations of 1x10-5 Molar was introduced in unlike concentration to 10 test tubes and to have later concentrations resembling 9x10-5 Molar, 8x 10-5 Molar, 7x10-5 Molar, 6x10-5 Molar, 5x10-5 Molar, 4x10-5 Molar, 3x10-5 Molar, 2x10-5 Molar, 1x10-5 Molar. Now solutions were rightfully mixed. Then absorbance rates of solutions were found at the 500 nm throughout UV spectrometer [9].
Using Disc Diffusion Process
Solution of commemorated concentrations (Like 3μg/ml) of test samples were completed during dissolving measured quantity of trials in calculated quantity of solvents. The dried in addition to sterilized filter paper discs (such as 6 mm as a diameter) are afterward impregnated with recognized amounts of trial matters by using micropipette. Discs holding experiment substances were placed on the nutrient agar media consistently seeded by the test organism, idiosyncratic antibiotic discs in company with blank discs were exploited as a positive as well negative control. These plates were kept at the low temperature like 4 oC for 24 hours to allow maximum diffusion. Throughout this moment in times desiccated discs drain off the water from the adjacent medium and trial samples liquefy along with the extensive of trial disc. The diffusion occurs according to the physical rule so as to manages diffusion of molecules during the agar gel. Therefore there was a steady alter of trial matters concentrations in the medium adjoining disc [10].
Results and Discussion
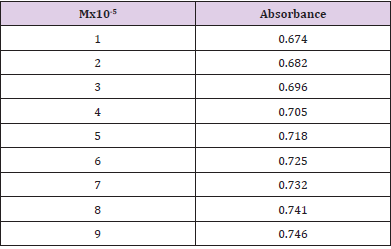
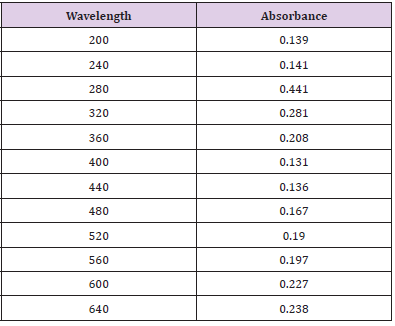
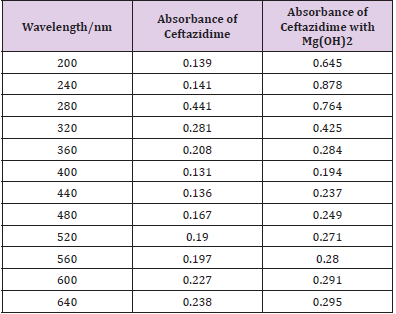
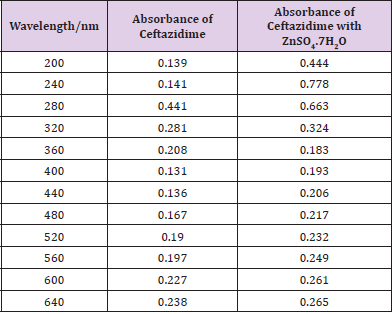
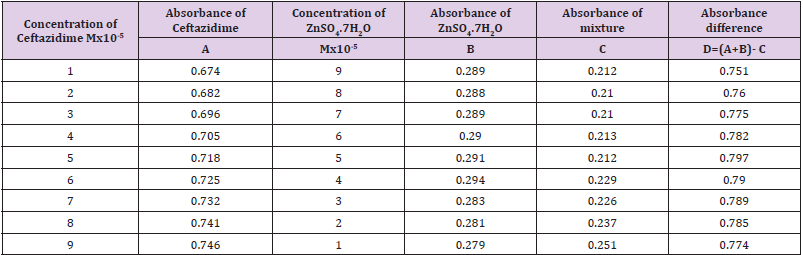
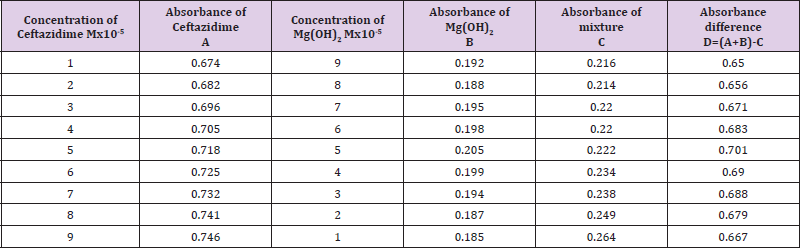
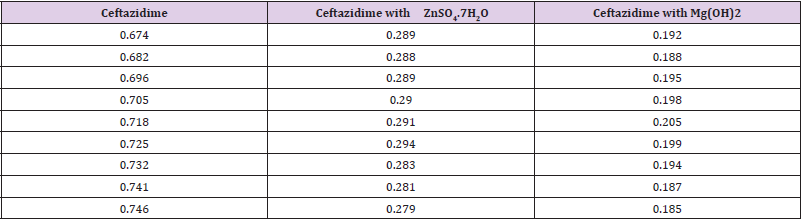
From the succeeding Tables 3 & 4, this can examine to absorbance of Ceftazidime enhances with enhancing the concentration in keeping with the Beer Lambert’s equation. From Table 5, it can monitor that absorbance of Ceftazidime is different and it interacts with Mg(OH)2. From Table 6, it can monitor that interaction between the drug and also metal may guide to form complexes which have dissimilar light absorption ability and spectrum pattern is also altered. So any change and spectrum actions is regarded as the tool for primary contact from spectral studies. Outcome of the metals on Ceftazidime by Job’s technique of incessant variation: Molar proportions of the complexes of metal salt were computed approximately through the Job;s method. The examined absorbance speeds were calculated in pH 7.4 at varied concentration such as 1x10-5 to 9x10-5 Molar of Ceftazidime by metal. Then the Job’s plots at pH were needed through plotting absorbance difference against mole part of drug (Table 7). From above we can monitor that Ceftazidime forms the strong 1:1 complexes with the zinc sulfate hepta hydrate which is designated as the inverted ‘V’ shaped curve (Table 8). From above we can monitor that Ceftazidime forms the strong 1:1 complexes with the Mg(OH)2 which is designated as the inverted ‘v’ shaped curve (Table 9).
Antimicrobial Reading
The antimicrobial efficiency of the trial representatives is calculated via their action to pass up the enlargement of the microorganisms neighboring recordings which provides the clear region of inhibition. Past incubation, the antimicrobial acts of the testing materials were finished through the measuring diameter of the zones of embarrassment inside millimeter throughout a very clear mm scale. The trial models were examined also in opposition to the Staphyloccopcus aureus. The typical Ceftazidime disk checked also in opposition to the Staphyloccopcus aureus. The outcomes of antimicrobial action, computed considering diameters of the zone of inhibition in mm were revealed in Table 10. Antimicrobial feeling testing of Ceftazidime against the Staphylococcus aureus later than interacting with the ZnSO4.7H2O and also Mg(OH)2 solution correspondingly.
Now it has proved that the zone of the inhibition of Ceftazidime with Metal and also antacid Zn, Mg diminished from the 16 mm to 14 mm and 15 mm correspondingly owing to the metal, used antacid and also drug interaction.
Conclusion
The classy spectrophotometric technique is very easy, straight in addition to worthwhile for fortitude of the drugs. Commencing this spectral interpretation, this has also been observed that Ceftazidime provides the pointed peak at 500 nm. At what time the Zinc Sulfate additionally antacid solution like Mg(OH)2 merged with Ceftazidime 1:1 ratio and also the strong point of peak alters tremendously and absorption characteristics are also changed owing to the interaction even though the position of the complex do not alter. Then the antimicrobial analysis of an intermediary is very crucial to observe its spectrum in opposition to a variety of natures of pathogenic microorganisms. The Job’s plot also have provided molar fraction of the complexes of Ceftazidime through the Zinc Sulfate as well as antacid solution such as Mg(OH)2. At pH 7.4 Ceftazidime structures sturdy 1:1 complexes throughout Zinc Sulfate plus antacid solution similar to Mg(OH)2 assigned as ‘^’ shaped curves. These curves also can indicate the wellbuilt kinetics of complexation between Ceftazidime by the Zinc Sulfate in company with antacid solution similar to magnesium hydroxide. The trial models were checked also in opposition to the Staphylococcus aureus. The typical Ceftazidime disk also ensured in opposition to the Staphylococcus aureus. It was also examined that the antimicrobial action of Ceftazidime reduces and it structures complexes throughout ZnSO4.7H2O as well as antacid solution similar to magnesium hydroxide. Thus, by the antimicrobial assessment, it was established that the zone of inhibition of the Ceftazidime with Metal and also antacid Zn, Mg diminished from the 16 mm to 14 mm and 15mm respectively.
References
- Gentry LO (1985) Antimicrobial activity,pharmacokinetics,thera-peutic indications and adverse reactions of ceftazidime. Pharmacotherapy 5(5): 254-267.
- Derbyshire PJ, Williamson PJ, Pedlar SJ, Speller DCE, Mott MG, et al. (1987) Ceftazidime in the treatment of febrile immunosuppressed children. J Antimicrob Chemother 12: 357-360.
- Fainstein V, Bodey GP, Elting L, Bolivar R, Keating MJ, et al. (1983) A randomized study of ceftazidime compared to ceftazidime and tobramycin for The treatment of infections in cancer patients. J Antimicrob Chemother 12: 101-110.
- Fong IW, Tomkins KB (1985) Review of Pseudomonas aenrginosa meningitis with special emphasis on treatment with ceftazidime. Rev Infect Dis 7(5): 604-612.
- Norrby SR (1985) Role of cephalosporins in the treatment of bac- terial meningitis in adults: overview with special emphasis on ceftazidime. Am J Med 79: 56-61.
- Lopez KJV, Bertoluci DF, Vicente KM, Aquilla AMD, Santos SRC J (2007) Simultaneous determination of cefepime, vancomycin and imipenem in human plasma of burn patients by high-performance liquid chromatography. J Chromatogr 860(2): 241-245.
- Denooz R, Charlier C (2008) Simultaneous determination of five β - lactam antibiotics (cefepim, ceftazidim, cefuroxim, meropenem and piperacillin) in human plasma by high - performance liquid chromatography with ultraviolet detection. Ibid 864(1-2): 161-167.
- Koppisett VS, Chandra N (2011) Influence of Alcohol and Smoking on Drug Action: A Step for better utilization of drugs. Journal of Chemical and Pharmaceutical Research 3(1): 242-248.
- Sadowski CD (2012) Drug Interactions with Antacids Mechanisms and Clinical Significance. springer international journal 11(6): 395-407.
- Billov S, Kizek R, Jelen F, Novotn P (2003) Square-wavevoltammetric determination of cefoperazone in abacterial culture, pharmaceutical drug, milk, and urine. Analytical and Bioanalytical Chemistry 377(2): 362-369.

 Research Article
Research Article