Abstract
Infection with Toxoplasma gondii, is one of the most widespread zoonoses in the
world. Congenital toxoplasmosis (CT) is particularly risky due to its fetal complications.
Global risk of CT transmission is approximately 40%, reaching 90% in the last month
of pregnancy. Children with CT frequently require treatment, usually in Argentina with
sulfadiazine (SDZ) and pyrimethamine (PYR), to prevent morbidity. Therapy for pediatric
patients is hampered by the absence of pediatric formulations. To address this problem,
SDZ and PYR are prepared as extemporaneous formulations by hospital pharmacies in
the form of syrups. At the moment, serological concentrations of these formulations
have not been corroborated in patient serum samples. The objective of this study was
to develop a bioanalytical method for identification and simultaneous quantification of
SDZ and PYR by high performance liquid chromatography (HPLC) with UV detection.
The validated method was tested with residual serum samples obtained from 6 pediatric
patients undergoing treatment with SDZ 42.20 a 93.70 mg/kg/day and PYR 0.77 a 2.70
mg/kg/day. Calibration curves were made for SDZ and PYR by spiking both drugs on
drug-free serum samples. Pretreatment consisted of a deproteinization step with
trichloroacetic acid followed by centrifugation and then injection of supernatant. Limit
of detection (LOD) and quantification (LOQ) were (0.17 ±0.02 and 0.13 ±0.02) μg/mL
and (0.46 ±0.01 and 0.36 ±0.01) μg/mL for SDZ and PYR respectively. The validated
method had a linear range of (< LOQ - 210.00 ±0.02) μg/mL for SDZ and (< LOQ - 15.05
±0.02) μg/mL. Serum samples range concentrations found were ( We developed a rapid, accurate, precise HPLC method for quantification of SDZ and
PYR simultaneously, using the most commonly employed C-18 column with UV detection
with sufficient sensitivity to be applied to therapeutic monitoring in pediatrics. It is the
first report of dosages of serum concentrations of SDZ and PYR in pediatric samples
carried out in public institutions in Argentina. Keywords: Toxoplasmosis; Bioanalytics;
Pediatric Pharmacology; Therapeutic Drug
Monitoring; Neglected Diseases Infection with Toxoplasma gondii, is one of the most
widespread zoonoses in the world. It is estimated that one third
of the world’s population is infected. The infection is acquired
mainly by food contaminated with parasite cysts and is usually
asymptomatic. The congenital infection is particularly risky,
with rates of mother to baby transmission approaching 90%
in the last month of pregnancy. There are few studies on the
incidence of congenital toxoplasmosis (CT) in Latin America,
but the seroprevalence in women of childbearing age is high [1].
Mostly the infection is asymptomatic, and the diagnosis is made
by serological screening. Treatment during pregnancy decreased
fetal morbidity and sequelae in the child [2]. An early diagnosis
followed by treatment of CT in infants provides a better resolution
of clinical signs compared to those not treated [3,4]. Between 10
and 30% of prenatal infections result in abortion, death of the
newborn or severe clinical signs at birth [5,6]. However, about 67%
of congenital infections are clinically asymptomatic at birth and
may develop symptoms later, predominantly ocular lesions [3,6].
The current therapy in pediatric patients is protocolized, but due
to the absence of pediatric formulations of the drugs, these are
prepared in the hospital pharmacy in the form of syrup and at the
moment, pharmacological parameters of these drugs have not been
locally corroborated in this population of patients, especially for
the combination of SDZ and PYR. A protocol designed to evaluate the response of a bioanalytical
method for identification and simultaneous quantification of SDZ
and PYR by high performance liquid chromatography (HPLC) with
UV detection was followed. The aim is to validate the HPLC-UV
method in order to transfer these capabilities to health institutions
that perform therapeutic monitoring of these drugs. Instrumental
techniques using HPLC-UV require equipment of medium
complexity suitable for the monitoring of pharmacotherapy,
available in hospitals and institutions of the public health system
in Argentina. Accurate standard solutions of SDZ, PYR and a mix of both of
them were evaluated at different concentration levels. Duplicate
samples of these standard solutions were stored in batches in
freezer and refrigerator respectively. After a defined period of
time - between 24 hours and 30 days of storage - each solution was
quantified. For every sample, its percentage coefficient of variation
(CV %) intra-day (repeatability) and inter-day (reproducibility)
was determined after its storage in refrigerator or freezer. Here,
there was no pretreatment step needed for these samples, so as to evaluate only the chromatographic system response and the stability
of these standard solutions. An analogous treatment was performed
for spiked serum samples at accurate and known concentrations of
SDZ, PYR and a mix of both. Stability concentration after storage
in the freezer between 24 hours and 30 days was also evaluated
for these samples. Here, a pretreatment was needed to extract SDZ
and PYR from the serum matrix. Percentage of recovery (R%) was
calculated for all stored samples and for a fresh spiked serum sample
with an accurate concentration as one of those stored, in every day
of analysis [10]. Finally, another stability evaluation was performed
in 4 serum samples after 3 cycles of defreeze / freeze at an accurate
and known concentrations of SDZ, PYR and a mix of both, followed
by extraction and quantification, according to protocols proposed
by the National Administration of Medicines, Food and Medical
Technology (ANMAT) for the stability of a bioanalytical method
[11]. It was proposed to take as a stable criterion those samples
that presented a CV % ≤ 15.0 % in all instances. Trichloroacetic acid (TCA) pro analysis grade was purchased
from Biopack (Buenos Aires, Argentina). Dimethyl sulfoxide (DMSO)
pro analysis grade was obtained from Anedra (Buenos Aires,
Argentina). Chromatographic grade demineralized water (<0.2
μsiemens) was obtained in our laboratory with ionic interchange
resins. Acetonitrile (AcN) and Methanol (Me) (J.T. Baker, USA) HPLCgrade
were used. Sulfadiazine (Stanton L_1205050015269/0088)
was obtained from the hospital pharmacy and pyrimethamine was
obtained from sigma Aldrich. A Tabletop centrifuge (MRC, Scientific Instruments, Argentina) and a rotary evaporator (Heidolph
Laborota 4010) equipped with a ROTAVAP valve control equipment
were used for the pretreatment procedures. A certified 0.1 mg
analytical (Ohaus-Pionner, USA) was used in weighing operations.
All micropipettes were calibrated before use. All HPLC solvents were
degassed with a vacuum pump (Pascal, Buenos Aires, Argentina).
An ultrasonic homogenizer (FAETA, Argentina) was also used on
extraction procedures. The instrumental analytical procedure for samples measures
were performed with an LC system consisting of an HPLC Merck-
Hitachi LC-6200A and Merck-Hitachi UV/Vis L-4250 detector
(Japan). Separations were carried out at room temperature using
a C18 column 5 μm, 100 mm ×4.6 mm I.D. Lichrospher-100 RP18
(Merck, USA). Samples were injected with a manual injector system
with a 20 μL sample loop. Peak areas were integrated automatically
by Merck-Hitachi D-2500 Chromato-Integrator. All the calculations
concerning the quantitative analysis were performed with an
external standardization by the measurement of peak areas of a
sample specimen series. Limits of detection (LOD) were established
at 3.3 times of intercept coefficient standard error/slope coefficient
ratio. Limits of quantization (LOQ) were established at nine times
of intercept coefficient standard error/slope coefficient ratio.
Accuracy and precision of the assays were calculated based on the
analysis of three replicates for each level of the standard curve. Total
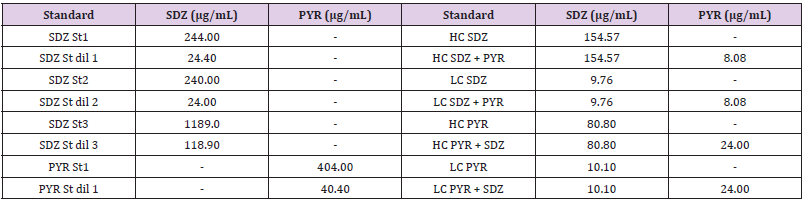
uncertainty was calculated as the sum of accuracy and precision. Table 1: Drug Standard Solutions evaluated in aqueous and serum matrix.SDZ St: Sulfadiazine Standard Solution; SDZ St dil: dilution
1/10 of Sulfadiazine Standard Solution; PYR St: Pyrimethamine Standard Solution; PYR St dil: dilution 1/10 of Pyrimethamine
Standard Solution; HC SDZ: High Sulfadiazine Concentration on serum; HC SDZ + PYR: High Sulfadiazine Concentration with
pyrimethamine on serum; LC SDZ: Low Sulfadiazine Concentration on serum; LC SDZ + PYR: Low Sulfadiazine Concentration
with pyrimethamine on serum. HC PYR: High Pyrimethamine Concentration on serum; HC PYR + SDZ: High Pyrimethamine
Concentration with sulfadiazine on serum; LC PYR: Low Pyrimethamine Concentration on serum; LC PYR + SDZ: Low Pyrimethamine
Concentration with Sulfadiazine on serum. Standard solutions of SDZ and PYR were prepared separately.
For SDZ standard solution, 0.0507g were dissolved in 10 mL of
DMSO. To complete dissolution it was accurately diluted to 25.00
mL in a calibrated volumetric flask with a solvent mix of Me:water
(50:50) to obtain a 2028.0 μg/mL SDZ solution. For PYR standard
solution, 0.0297 g were dissolved in 10 mL of AcN and then
accurately diluted to 25.00 mL in a calibrated volumetric flask
with the same solvent mix used for SDZ to obtain an 1189.0 μg/
mL PYR solution. These standard solutions and dilutions of them
were used for stability studies (Table 1). Also, variable volumes of
the described standard solutions of SDZ and PYR were added to
drug-free serum to obtain matrix standards of 1,000 μL volume for calibration curves. These curves were made in triplicate for
the lowest concentration point and duplicated for the rest of the
points. Duplicated drug-free serum samples as a control specimen
were included on calibration curves. Concentration points for both
drugs analysed were (0.05; 0.56; 5.60; 70.50; 170.10; 240.66) μg/
mL for SDZ and (0.03; 0.51; 0.91; 2.42; 7.07; 16.10) μg/mL for PYR
(Table 2). In all cases the volume of the standard solution added to
the serum matrix did not exceed 20% off its volume to minimize
dilution effects. For control specimen a 20% serum sample volume
of the solvent used for standard solutions was added. Table 2: Concentration points used for calibration curves. CS:
Control Specimen; St 1 to 6: Standard points at six concentration
levels; a/b/c: indicates triplicate samples; a/b: indicates
duplicate samples. Residual serum samples were obtained from six pediatric
patients treated for TC from a clinical study with PYR (0.77 a
2.70 mg/kg/day), aged between 33 days and 3-year-old. Samples
were stored at –20◦C until analysis. The clinical study and its
informed consent for the use of the samples were approved by the
institutional ethics committee of the Ricardo Gutiérrez Children’s
Hospital (HNRG). All 1,000μL samples were deproteinized with 50 μL of TCA
(30% p/v), vortexed for one minute, and sonicated for five minutes.
The mixture was then centrifuged at 8000 g for another five 5
min. After this, 300μL of the supernatant were separated on an
eppendorf before injecting it into the HPLC system. The HPLC analysis was performed by a gradient elution in a reverse phase
(RP) mode. The mobile phase composition varied from 90 to 50%
of water (1% v/v of formic acid) with methanol from 5 to 45% and
5% of acetonitrile that remained constant throughout the run. The
flow ranged was 0.8 to 1.0 mL/min and the total running time was
fourteen minutes. All solvents were filtered through a 0.45μm nylon
membrane and degassed before use. The maximum UV absorption
found for simultaneous identification of SDZ and PYR was at 273
nm, so this wavelength was chosen for the method. A value of 0.030
absorbance units (a.u.) threshold was used. Duplicate injections
were made for all samples to test reproducibility of the detector
response at each concentration level. Peak area was plotted against
concentration to obtain calibration graphs. Linear regression and an
analysis of variance (ANOVA) were applied to calculate calibration
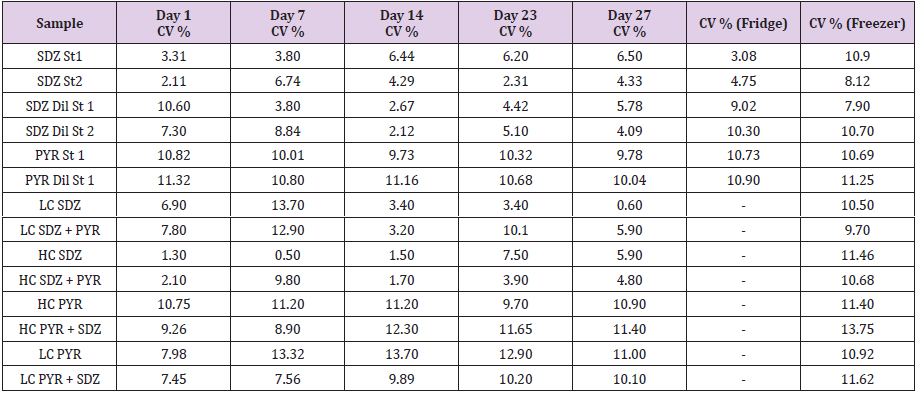
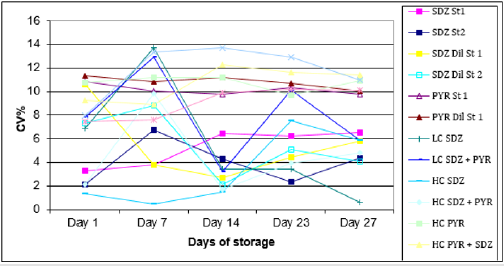
equation and statistical correlation coefficients. Table 3 and Figure 1 shows intraday CV % (repeatability),
between duplicates of standard solutions in refrigerator and freezer
after each storage time. The last two columns of the table present
the CV% inter-day (reproducibility) for each standard in solvent
or in serum for storage in refrigerator or freezer respectively.
Higher concentrated standard solutions presented lower CV%
than those diluted. Although the reproducibility in no case exceeds
the maximum CV% proposed as acceptable, it was found that the
standards stored in the refrigerator within the period evaluated,
have lower CV% than those stored in the freezer. This variation
may be due to factors such as the decrease in the solubility of
drugs at low temperatures associated with some systematic error
in the homogenization prior to injection into the chromatographic
equipment. No evaluation on serum was made for sample storage
in the refrigerator because it is well known that serum samples
refrigerator storage is not recommended for periods longer than 24
hours. For this reason and not to add an extra instability factor, only
its behavior stored in freezer was studied. For these serum samples,
there were no significant differences in CV% or R% between high
or low concentrations of both SDZ and PYR. Also, the presence
of the two drugs together in the matrix did not cause analytical
interference or significant increases in its CV% or %R. Table 3: Sulfadiazine and Pyrimethamine Stability over time. Columns 2 to 5 shows intra-day (repeatability) percentage coefficient
of variation (CV %) between duplicates in refrigerator and freezer after each storage time. The last two columns shows inter-day
(reproducibility) CV% for storage in refrigerator or freezer respectively. Sample References were presented on Table 1. Figure 1: Intra-day CV % (repeatability) between duplicates of standard solutions in refrigerator and freezer after each storage
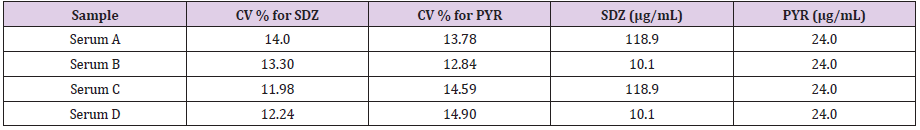
time. The CV % obtained for serum samples after 3 cycles of defrosting
/ frizzing are presented in Table 4. There, it can be observed that
the CV% are slightly higher for PYR than for SDZ (average CV% of
12.88 for the SDZ and 14.03 for the PYR) but they do not exceed
the values proposed as acceptable in any case. Calibration curves
for simultaneous detection and quantification of SDZ and PYR
were made on three different days with six concentration levels.
Retention time range for gradient mode used on chromatographic
analysis was (5.01-5.36) minutes for SDZ and (12.16-12.60)
minutes for PYR. Also, SDZ and PYR retention time ratio had a range
of 0.41- 0.43 and a total run time was 14 minutes per sample. The
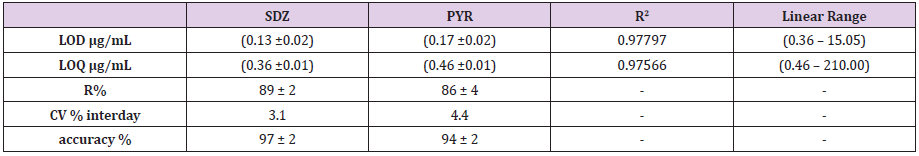
LOD and LOQ were (0.17 ±0.02 and 0.13 ±0.02) μg/mL and (0.46
±0.01 and 0.36 ±0.01) μg/mL for SDZ and PYR respectively. The
validated method had a linear range of (< LOQ – 210.00 ±0.02) μg/
mL for SDZ and (< LOQ - 15.05±0.02) μg/mL for PYR. A summary
of calibration curve statistical parameters is presented on Table 5.
Chromatographic parameters such as resolution, selectivity, and
peak asymmetry were satisfactory for SDZ and PYR determination
with this method. In addition, analytes showed no decomposition
products detectable in chromatograms profiles. Figure 2 presents a
characteristic chromatogram profile for the proposed RP gradient
chromatographic system for the detection and quantification of
SDZ and PYR in Table 4: The CV% obtained for serum samples at different concentrations after 3 cycles of defrosting / frizzing are presented here. Table 5: Statistical parameters found for simultaneous calibration curve for SDZ and PYR: LOD: Limit of detection; LOQ: Limit
of quantification; R (%): Percentage of recovery; CV % interday: inter-day (reproducibility) percentage coefficient of variation; R2:
R-squared value. Figure 2: Characteristic chromatogram for the validated method for SDZ and PYR quantification in a) standard solutions With the validated method, SDZ and PYR were measured in
a set of 30 samples from 6 pediatric patients who participated in
a clinical study to evaluate the pharmacokinetics of both drugs
on serum samples. The concentrations range found were ( The two drugs analytically evaluated on this study SDZ and
PYR, are currently available for the treatment of TC on public
health Institutions in Argentina. As the fact that an appropriate
pediatric formulation is not commercially available, administration
of SDZ and PYR requires to be prepared in the hospital pharmacy
in the form of syrup. Actually, serological concentrations of these
formulations have not been corroborated in patient serum samples
before. Until now, there were no validated methods by HPLC/
UV developed for the simultaneous detection of both drugs. In
this sense, it is important to remark that with this method it was
possible to corroborate stability of these drugs on different matrices
and its dependence on sample storage period between fridge and
freezer. This proves that the robustness of the method is suitable
for therapeutic monitoring, pharmacokinetics and toxicokinetics. In literature, there is a developed method for determination of
PYR, sulfadoxine, mefloquine, and ibuprofen by HPLC/UV for
determination of these drugs in raw materials and dosage of
pharmaceutical formulations but SDZ was not included [12]. Also,
there are methods developed for SDZ and its hydroxy metabolite
and its quantification by reverse phase HPLC [13], and there are
others for PYR by HPLC and fluorescence detection but applied to
the malaria pharmacotherapy [14] or for TC but with sulfadoxine
instead of SDZ also by HPLC/UV. There is an interest in the determination of clinically significant
serum range of SDZ and PYR concentrations. To our knowledge,
there are two different scenarios: adult serum concentration and
pediatric serum concentrations. The LOQ of the method described
here for both SDZ and PYR, seems to be appropriate in pediatric
contexts. Most of the pediatric samples obtained were of 1.00
mL or less volume of serum. In this sense, minor sample volume
may imply a decrease in the sensibility of the method, so there is
a compromise between these two variables, also attending that
blood samples volumes in pediatrics are normally smaller than in
adults. It is important to note that no extra samples were taken for
the evaluation of the method developed from the pediatric patients
because residual volumes of serum were taken from an ongoing
clinical study. The development of HPLC methods for determination
of drugs has received considerate attention in recent years because
of their importance in the quality control of drugs and drug
products. The goal of this study was to develop a rapid, accurate,
precise, and less time-consuming HPLC method for analysis of SDZ
and PYR simultaneously, using the most commonly employed C-18
column with UV detection. A rapid, precise, accurate, low-cost, RP-HPLC-UV method
for simultaneous identification and quantification of SDZ and
PYR was developed, validated and tested its applicability on real
samples. The results are accurate and precise, confirmed by the
statistical parameters. The proposed method allows simultaneous
determination of both drugs with sufficient sensitivity to be
applied to therapeutic monitoring in pediatrics. It is the first report
of dosages of serum concentrations of SDZ and PYR in pediatric
samples carried out in public institutions in Argentina. The authors would like to gratefully acknowledge the
financial support and the doctoral and postdoctoral scholarships received from Consejo Nacional de Investigaciones Científicas y
Técnicas (CONICET), Agencia Nacional de Promoción Científica y
Tecnológica (ANPCyT) and Comisión de Investigaciones Científicas
de la provincia de Buenos Aires (CICpBA). The clinical study protocol and its informed consent for the
use of human samples were approved by the institutional ethics
committee of the Ricardo Gutiérrez Children’s Hospital.Introduction
Drugs available for the treatment of toxoplasmosis only inhibit
the growth of the parasite when it is in the active phase of its life
cycle (tachyzoite), not being useful against the cystic or latent form
of the parasite (bradyzoites). Most health centers do not hesitate
to recommend treatment to infants with confirmed CT. However, to
date, there is no controlled study in our country that determines its
efficacy, the appropriate therapeutic dose and the optimal duration
[6]. Indeed, there is a coincidence about the drugs to be used, but
the duration of treatment has been more discussed. Prolonged
treatments are associated with a lower rate of sequelae while
short treatments have the advantage of reducing drug toxicity.
The treatment scheme in Argentina is SDZ 50-100 mg / kg / d
associated with PYR 1 mg/kg/d and folinic acid 5 mg / 48 hours.
The duration is from diagnosis to one year of age with a minimum
time of 6 months if the child is older. PYR (5-(4-Chlorophenyl)-6-
ethyl-2,4-diaminopyrimidine) interferes with the synthesis of folic
acid by inhibiting dihydropteroate synthase and dihydrofolate
reductase and due to poorly studied pharmacological factors,
treatment may not be successful. SDZ (N-amino-N-pyrimidin-2-
yl-benzenesulfonamide) is the most active sulfamide against T.
gondii. It has synergistic activity with PYR but being analogous to
the PABA, necessary for the production of parasitic nucleic acids.
It is excreted by the kidney, requiring dose adjustment in patients
with renal impairment. It is not indicated in patients with glucose
deficiency 6-phosphate dehydrogenase (G6PDH) and replaced with
clindamycin.
In other countries there are few studies in the pediatric
population where PYR and SDZ on serum samples are quantified
but they are framed in populations in which different drugs
and combinations of drugs are used with different therapeutic
and combination of drugs used [7-9]. A publication describing
pharmacokinetic parameters for pediatric population treated
several months for CT with PYR and sulfadoxine, proposes
the existence of a wide interindividual variability and that at
a dose adjusted to weight, plasma concentrations would be
unpredictable. Therefore, it has not been possible to establish
what plasma concentration in the combination of drugs is most
effective in pediatrics. The relationship between therapeutic blood
concentrations and toxicity is unknown and there are also no
studies of interaction with new anticonvulsants or corticosteroids.
On the other hand, the transfer information of these drugs through
the placenta or breast milk is scarce. All these vacancies translate
into important working hypotheses. Despite addressing drugs with
a long time of use in therapeutics, there is no sufficient information
in the literature in the pediatric field. To advance in any of the
hypotheses, a simple, fast, precise and clinically adjusted method,
such as the one presented in this work, is of great importance
as a tool for systematization and improvement of the current
pharmacological treatment protocols for this disease.Methods
SDZ and PYR Stability Over Time
Materials and Reagents
HPLC Instrumentation and Calculation
Standard Solutions
Serum Samples
Sample Pretreatment and Chromatographic Conditions
Results
a) standard solutions mix.
b) serum extracts for SDZ and PYR.
b) serum extracts for SDZ and PYR.SDZ And PYR on Pediatric Samples
Discussion
Instrumental techniques using HPLC-UV require equipment of
medium complexity suitable for the monitoring of pharmacotherapy,
available in hospitals and institutions of the public health system,
giving its advantages over others reported HPLC methods for
determination of some of these two drugs but through more
expensive and sophisticated detection systems [15].Conclusion
Funding and Acknowledgements
Compliance with Ethical Standards
References

 Research Article
Research Article