Abstract
Coronavirus disease (COVID-19) is caused by SARS-COV2 and represents a global public health problem. E l use of ivermectin for the treatment of SARS-COV2 the World Health Organization encourages concerns the use of therapy untested in the context of randomized clinical trial. Azithromycin has shown success in the SARS-COV2 . The low molecular weight heparins have decreased mortality COVID-19, and l Rivaroxaban is an alternative pathway oral for use. E l objective was to evaluate the percentage of patients diagnosed with COVID-19 that modify their clinical evolution under treatment comparative early intervention.
Material and Methods: Design randomized experimental, single - blind, prospective, longitudinal and open in 114 patients with COVID-19 UMF No. 13 and No. 20 of November to December 2020 , prior informed consent is given medication randomized 67 patients (Azithromycin / Ivermectin a / Ribaroxaban ) vs. 47 (Azithromycin / Ribaroxaban ); followed by video call for 14 days with evaluation of the outcome of clinical symptoms such as headache, cough, fever, conjunctivitis, myalgia, arthralgia, rhinorrhea, odynophagia, anosmia, chest pain, dyspnea, using statistics with Student’s t test , survival tables, using SPSS version 21.
Results: There was an improvement in the modification of the clinical evolution of the symptoms of patients with COVID-19 with double therapy in 95.7% (n = 44) vs. 90.8% with triple therapy (n = 59) with therapeutic failure in 4.3 % (n = 2) vs. 9.2% (n = 6) respectively, with a value of p = 0.327.
Keywords: COVID-19; Outpatient Follow-Up Telemedicine; Early Intervention Treatment
Introduction
In March 2020 the World Health Organization declares the COVID-19 disease as a Pandemic [1]. In Mexico there are with COVID-19 until March 2021, 2, 151, 028 confirmed patients, 440,983 suspected cases and 193,142 deaths [2]. The incubation period for COVID-19 is 5-6 days with an average time between the onset of symptoms and recovery in patients with mild illness of approximately 2 weeks [3,4]. Fever, cough and headache are the most common symptoms in Mexico of COVID-19 [5]. Fever, cough, and headache are the most common symptoms of COVID-19 in Mexico. Ivermectin in in vitro studies has an antiviral effect in response to SARS-CoV-2 due to the fact that it presents pulmonary concentrations above its mean effective concentration maximum (EC50) in more than 10 times [6]. Within the pharmacokinetic action of absorption and elimination of Ivermectin as a possible treatment of COVID-19, they have been studied under a dose of 6mg of Ivermectin. Various studies have been conducted on the efficacy of Ivermectin in COVID-19 [7-9], but the results are not conclusive, as Lespine refers, mentioning to carry out more research to better evaluate the effectiveness of Ivermectin in the treatment of SARSCoV- 2 [10]. Authors report that Azithromycin has in vitro effects on SARS-CoV-2 [11,12]. In addition, the mechanism of action is like that of an anti-inflammatory drug, reducing the mediators of the secretory phenotype associated with senescence (SASP), such as IL-1beta and IL-6 [13,14].
In a Randomized Clinical Trial where dual therapy was given that included Azithromycin, 83% of the virologically cured patients were shown on day 4 of treatment of patients with COVID-19 [15]. Studies derived from COVID-19 conclude that initial treatment with Low Molecular Weight Heparins (LMWH) reduces mortality by 48% at 7 days and 37% at 28 days and achieves a significant improvement in the blood pressure / oxygen ratio. Inspired fraction of O2 (PaO2 / FiO2) by mitigating microthrombus formation and associated pulmonary coagulopathy. For this reason, the studies derived from COVID-19 use LMWH in all cases during admission in prophylactic doses for at least 7 days [16]. The use of Rivaroxaban 10 mg once daily has been compared with LMWH in the prevention of VTE (venous thromboembolism) after major orthopedic surgery [17]. Rivaroxaban is potent, oral, highly selective, a direct factor Xa inhibitor, and effective for primary and secondary thromboprophylaxis, [18,19] therefore, the hypothesis of this study is whether there will be a modification in the clinical evolution of ≥ 25% of patients with a diagnosis of COVID-19 under an early intervention treatment with Azithromycin / Ivermectin / Ribaroxaban vs. Azithromycin / Ribaroxaban for 14 days followed by video call from U.M.F 13 and U.M.F 20 from I.M.S.S.
Materials and Methods
The present study is a randomized, single-blind, prospective, longitudinal, and open-label experimental study design. Prior acceptance of informed consent, patients who belonged to Family Medicine Unit No. 20 and No. 13 of the Federal District were selected. North of the IMSS, with inclusion criteria that are patients over 18 years of age, with comorbidities such as Type 2 Diabetes Mellitus, Systemic Arterial Hypertension, Overweight or Obesity as well as meeting the operational definition COVID-19 and confirmatory test of P.C.R. positive within the first days of the disease (being evaluated in the Family Medicine Units), for the follow-up video calls to the patients UMF No. 20 and No. 13 there was installation of electronic equipment for the use of the Internet, Exclusion criteria are patients with severe COVID-19 (requiring immediate hospital referral), presence of any personal pathological history of hematological diseases, allergy to macrolides (Azithromycin) and Ivermectin.
Procedure
If the patient, with prior informed consent, agreed to participate in the study after the positive COVID19 test, patients with Mild Clinical Phase Covid-19, they were instructed to take medications according to the groups that will be managed and according to those prescribed by the doctor Researcher, it could be previous randomization assigned to Group A with taking the following medications: Paracetamol 500mg orally 1 tablet every 8 hours for 3 days in case of fever equal to or greater than 38.3 °C, Azithromycin 500mg tablets will take 1 tablet single dose the first day and then half a tablet (250mg) orally every 24 for 4 days, Ivermectin tablets of 200mcg which will be calculated according to your weight and dose, will be every 24 hours for 2 days and Rivaroxaban tablets of 10mg will take 1 every 24 hours for 10 days. If it was Group B, take Paracetamol 500mg orally 1 tablet every 8 hours for 3 days in case of fever equal to or greater than 38.3 °C, Azithromycin 500mg tablets will take 1 tablet single dose the first day and then half a tablet via orally every 24 hours for 4 days and Rivaroxaban 10mg tablets will take 1 every 24 hours for 10 days. Likewise, during the taking of medications and later until completing 14 days, a monitoring was carried out through a video call via WhatsApp, daily from 10am to 2pm, including Saturday and Sunday with a duration of approximately 15 minutes, to know and record the presence or absence of clinical symptoms (Headache, Cough, Fever, Conjunctivitis, Myalgia, Arthralgia, Rhinorrhea, Odynophagia, Anosmia, Chest pain, Dyspnoea) and adverse reactions (Diarrhea, Nausea, Vomiting, Disorientation, Dizziness, Asthenia, Ecchymosis / petechiae, Vertigo, Tinnitus, Urticaria, Hemorrhage, Angioedema, Palpitations, Chest pain) to medications related to COVID19. During the first day of your participation in the protocol, you will be informed to attend the General Hospital of Zone No. 48 of the IMSS at 8:30am, in the laboratory area for the extraction of 5 milliliters of blood and take a hematic biometry, C-reactive protein, D-dimer, ferritin, prothrombin time, thromboplastin time, and lactic dehydrogenase.
Statistics
For the size of the sample, it is stated that assuming an efficacy of 25% in modifying the clinical evolution (Symptoms of fever, cough, headache, myalgia, odynophagia, anosmia, rhinorrhea, arthralgia, chest pain, dyspnea, conjunctivitis) with a diagnosis of COVID-19 under a comparative early intervention treatment for 14 days followed by video call, with a power of 90%, a type I error rate of 1% and a loss to follow-up of 20%; We calculated a total of 114 patients with COVID-19, that is, 67 cases in group A with Azithromycin / Ivermectin / Ribaroxaban / Paracetamol treatment and 47 in group B with Azithromycin / Ribaroxaban / Paracetamol treatment would be necessary for the analysis. Statistical differences were evaluated using Pearson’s Chi-square test as categorical variables, Student’s t test for quantitative variables, survival analysis, and Kaplan-Meier using the log-rank test method, as appropriate. The analyzes will be carried out in SPSS version 21.
Randomization
For the conformation of the groups, random numbers generated by lottery were used in which the patient, as he arrived, took a piece of paper which had the number of the group to which it will be assigned. Performing a type of randomization by blocks, A (Azithromycin, paracetamol, Ivermectin and Ribaroxaban) and B (paracetamol, Azithromycin, and Ribaroxaban), with 65 and 46 participants, respectively. The mechanism used to implement the random assignment sequence was the use of two containers that will include 67 blue boxes and another of 47 orange boxes with the identification of the color to the assigned treatment, which was carried out by a doctor. The personnel who generated the random allocation sequence was a physician (considered the only role in the study) and who selected the participants and assigned the participants to the interventions was an associate investigator (considered the only role in the study). The study was kept blinded after assigning the interventions to the given patient who does not know what treatment he is receiving and to the treatment group A or B that was assigned.
Table 1: Characteristics population in patients with COVID-19 under a treatment of intervention early in the UMF 13 and UMF 20.
Note: *p <0.05 Pearson’s Chi- square test
Table 2: Cross tabulated outcome in modification of the evolution clinical vs fails therapeutic by type of treatment in patients with COVID-19 UMF 13 and UMF 20 of the IMSS.
Note: *p <0.05 Pearson’s Chi-square test
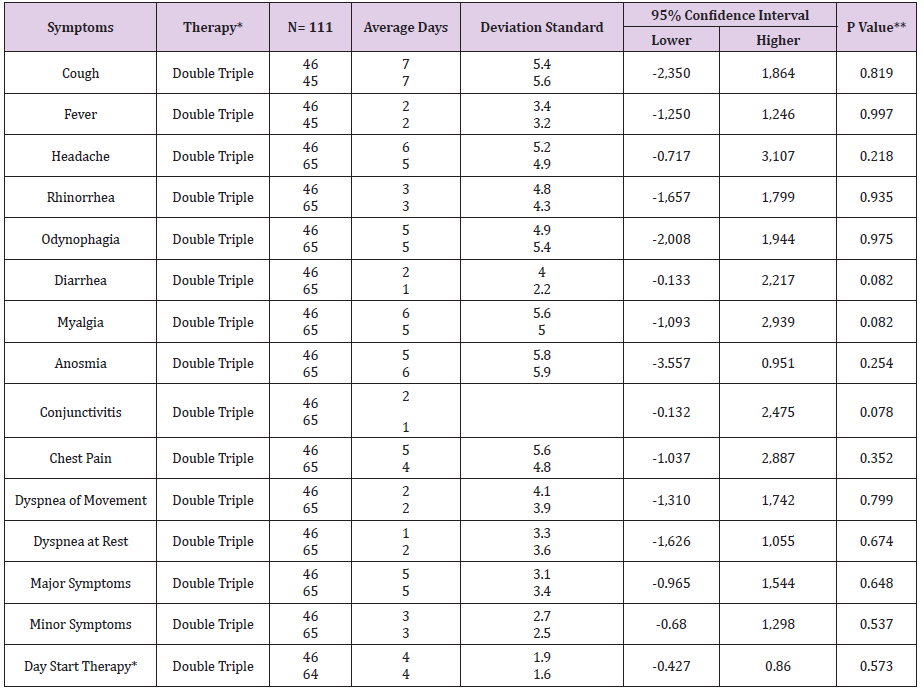
Table 3: Average days in COVID-19 symptoms by type of therapy in the UMF 20 and UMF 13 of the IMSS.
Note: *Double Therapy (Azithromycin, Rivaroxaban), Triple Therapy (Ivermectin, Azithromycin, Rivaroxaban) **p <0.05 , student’s t test
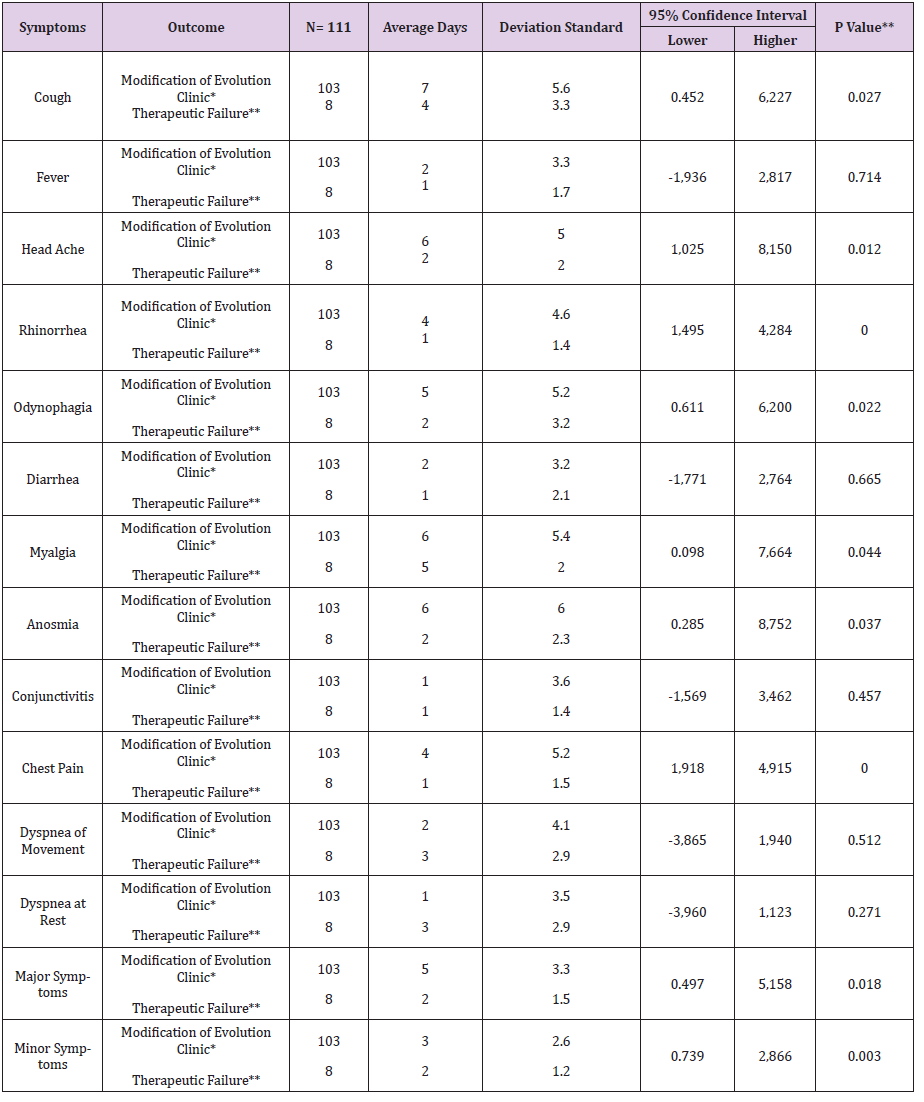
Table 4: Average days symptoms of COVID-19 under treatment intervention or n temp r ana for outcome in UMF 13 and 20 of the IMSS.
Note: *first level recovery , **shipment to second level, hospital. ** *p <0.05, student’s t test
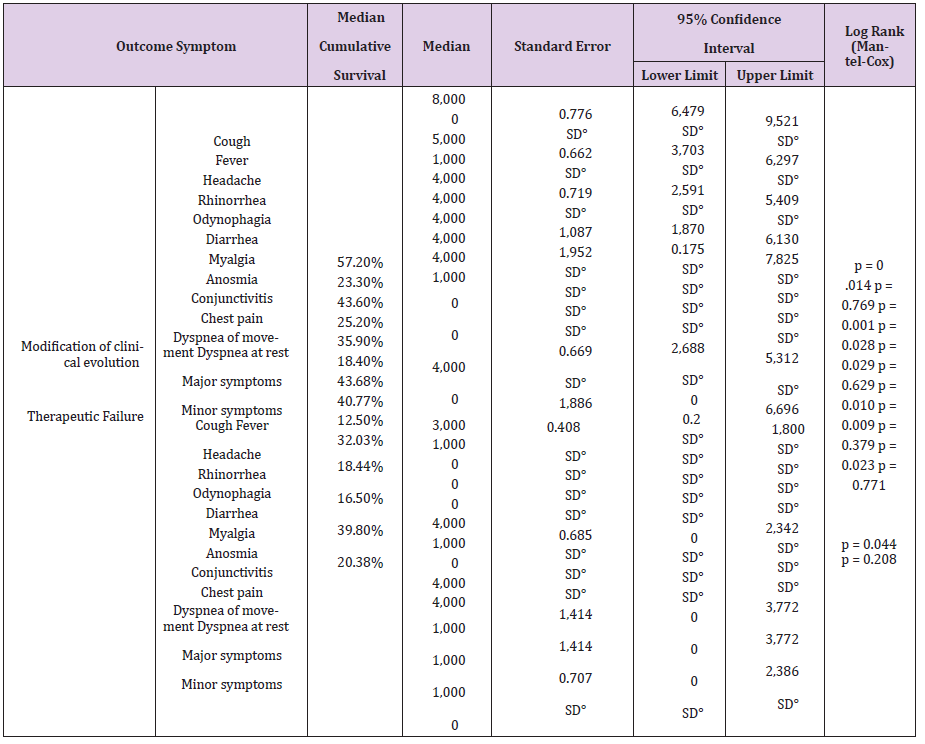
Table 5: Median accumulated survival in the days of COVID-19 symptoms under pharmacological treatment by outcome in the UMF 13 and UMF 20 of the IMSS.
Note: °No data
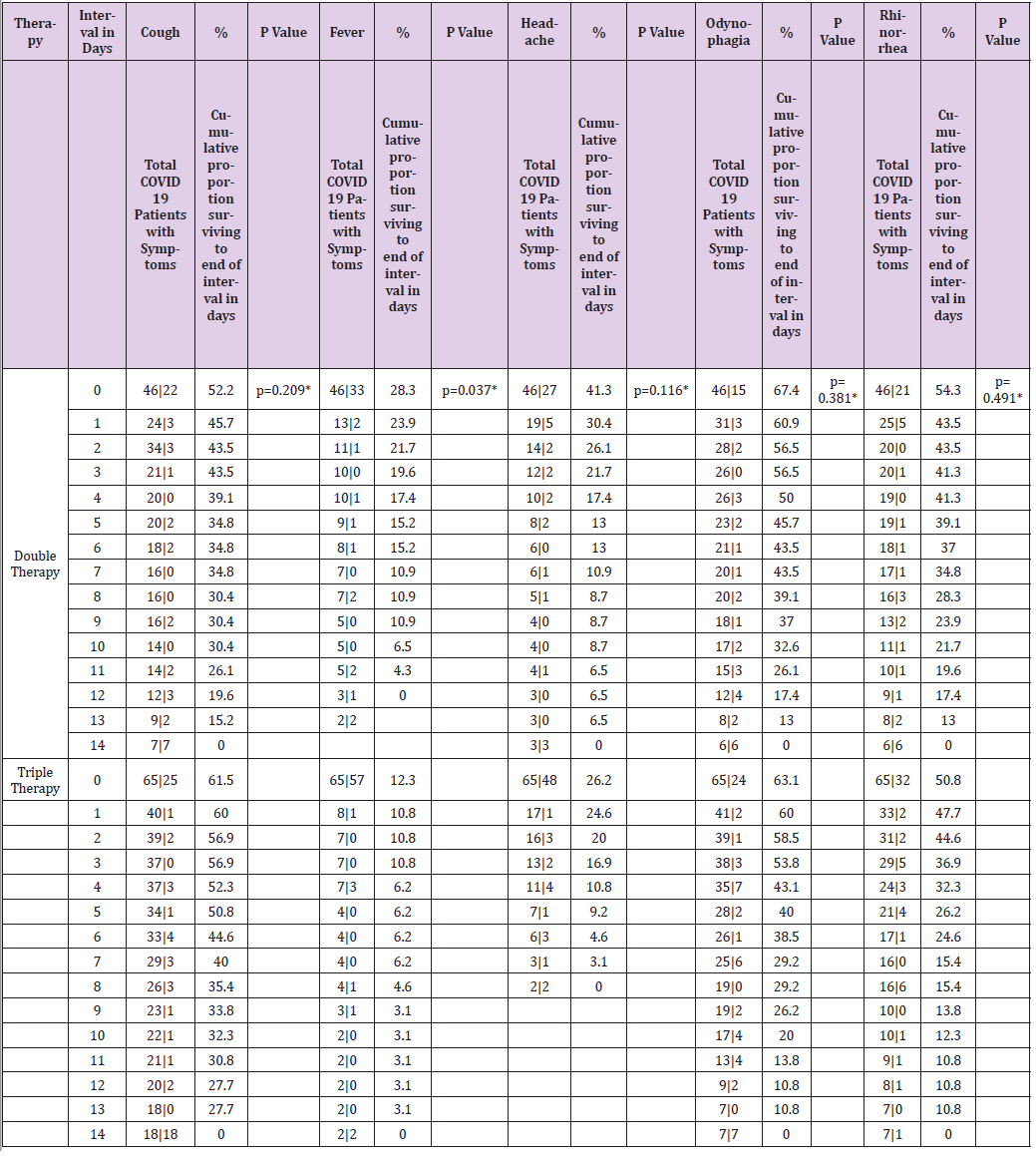
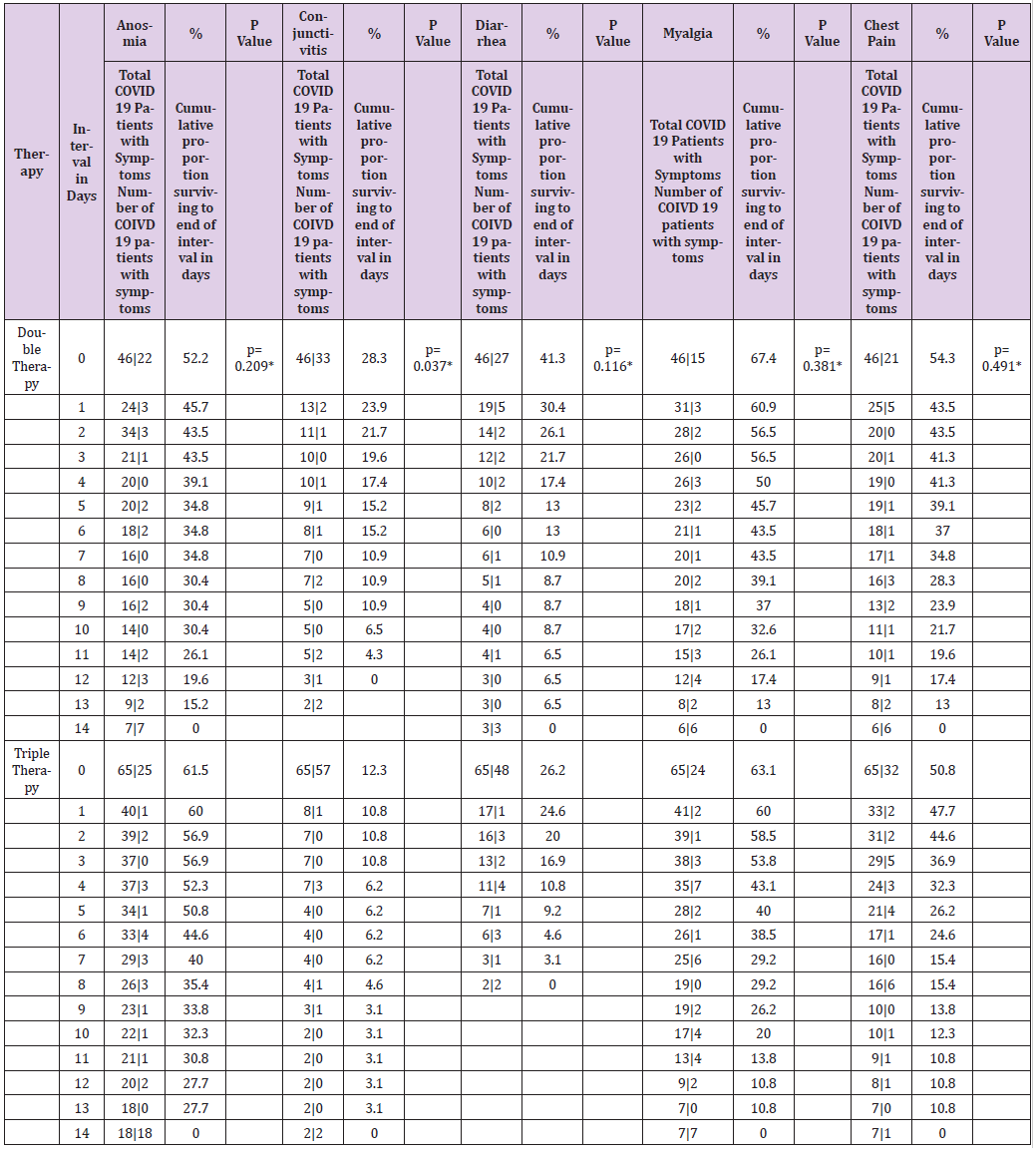
Table 6.1: Table of life of the symptoms of patients with COVID-19 by type of therapy during a follow-up for 14 days.
Note: therapy* Double Therapy ( Azithromycin, Rivaroxaban ), Triple Therapy ( Ivermectin, Azithromycin , Rivaroxaban ); Day and median survival of COVID-19 symptoms by type of therapy. * Statistically significant value p <0.05 Wilcoxon ( Gehan ) statistical test
Table 6.2: Table of life of symptoms of patients with COVID-19 by type of therapy during a follow-up for 14 days.
Note: therapy* Double Therapy ( Azithromycin, Rivaroxaban ), Triple Therapy ( Ivermectin, Azithromycin , Rivaroxaban ); Day and median survival of COVID-19 symptoms by type of therapy. * Statistically significant value p <0.05 Wilcoxon ( Gehan ) statistical test.
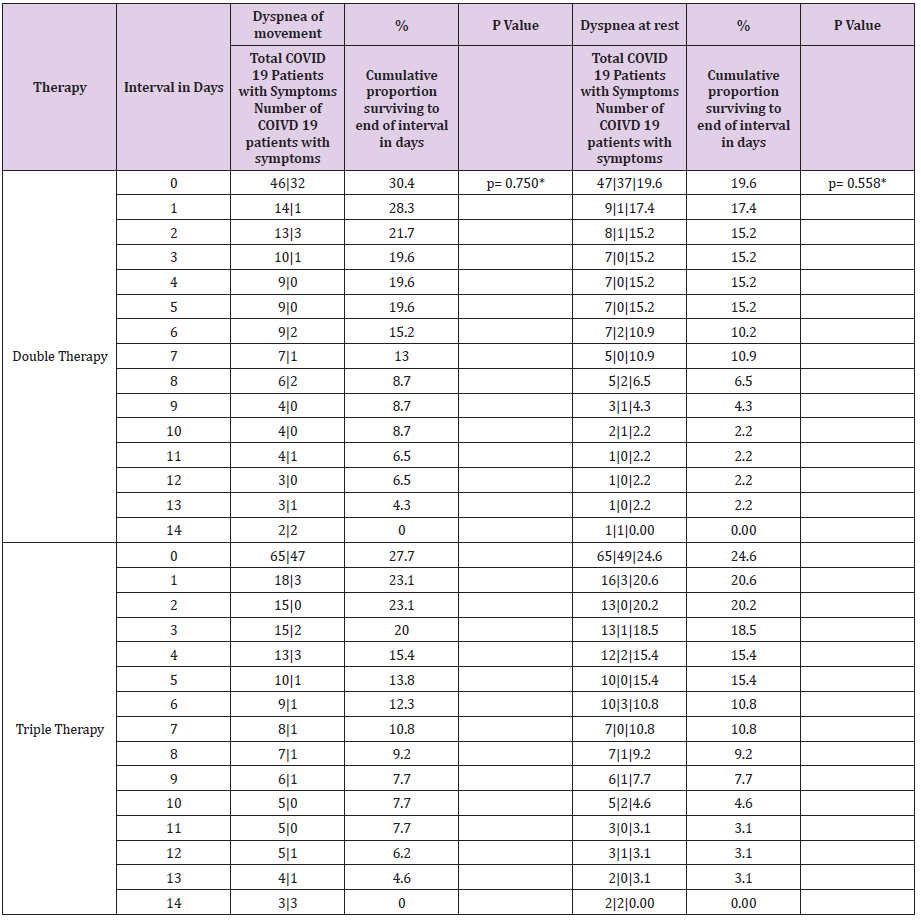
Table 6.3: Table of life of symptoms of patients with COVID-19 by type of therapy during a follow-up for 14 days.
Note: °No data
In the present study of the 114 patients to be eligible, 111 patients with COVID-19 who received early intervention double (n = 46) and triple (n = 65) therapy were analyzed (Figure 1); predominantly female with 52.2% (n = 24) vs. 52.3% (n = 34) respectively, with p value = 0.989. For both early intervention therapies, the age group 41-50 years predominated with 30.4% (n = 14) vs. 33.8% (n = 22), p = 0.471; Marital status Married with 54.3% (n = 25) vs. 67.7% (n = 44), p = 0.597; full-time occupation with 89.1% (n = 41) vs. 61.5% (n = 40), p = 0.032; the degree of undergraduate education with 45.7% (n = 21) vs. 49.2% (n = 32), p = 0.216 (Table 1). The outcome of improvement in the Modification of the clinical evolution of the symptoms of patients with COVID-19 using double therapy was reported 95.7% (n = 44) Vs. triple therapy 90.8% (n = 59) and therapeutic failure 4.3% (n = 2) vs. 9.2% (n = 6), respectively with p-value = 0.327 (Table 2). In relation to the presence of the number of days with clinical symptoms of COVID-19 by double therapy vs. triple therapy, there were no significant differences between symptoms. When comparing both therapies with the day of onset of the COVID-19 disease, an average of day 4 is reported with SD 1.9 vs. 1.6 respectively, p = 0.573 (Table 3). The average duration of days with clinical symptoms of COVID-19 under early intervention treatment by outcome in the improvement of the modification of the clinical evolution of symptoms vs. therapeutic failure, it is reported for the symptom Headache 6 days (SD 5.0) vs. 2 days (SD 2.0), p = 0.012; Cough 7 days (SD 5.6) vs. 4 days (SD 3.3), p = 0.027; Rhinorrhea 4 days (SD 4.6) vs. 1 day (SD 1.4), p = 0.000; Odynophagia 5 days (SD 5.2) vs. 2 days (SD 3.2), p = 0.022; Myalgia 6 days (SD 5.4) vs. 2 days (SD 2.0), p = 0.044; Anosmia 6 days (SD 6.0) vs. 1 day (SD 2.3), p = 0.037; Chest pain 4 days (SD 5.2) vs. 1 day (SD 1.5), p = 0.000; Symptoms greater than 5 days (SD 3.3) vs. 2 days (SD 1.5), p = 0.018; Minor symptoms 3 days (SD 2.6) vs. 2 days (SD 1.2), p = 0.003; respectively (Table 4).
The time it takes 50% of patients with COVID-19 for improvement in the modification of the clinical evolution of symptoms during a 14-day follow-up, the Cough symptom presented a median of 8 days, 95% CI (6,479 - 9,521) with a median cumulative survival of 57.2%, Log Rank test (Mantel-Cox) p = 0.014; Myalgia 4 days, 95% CI (1,870 - 6,130) with 43.6% cumulative survival, p = 0.010; Symptoms greater than 4 days, 95% CI (2,688 - 5,312) with 39.8% cumulative survival, p = 0.014; Odynophagia with a median of 4 days, 95% CI (2,591 - 5,409) with 35.9% cumulative survival, p = 0.029; Anosmia 4 days, 95% CI (0.175 - 7.825) with a 40.7% cumulative survival, p = 0.009; Headache 5 days, 95% CI (3,703 - 6,297) with 43.6% cumulative survival, p = 0.001; Rhinorrhea 1 day, with 25.2% cumulative survival, p = 0.044; Chest pain 1 day, with 32.03% cumulative survival, p = 0.023 (Table 5). The time it takes for 50% of COVID-19 patients to improve symptoms during a 14- day follow-up with dual therapy vs. triple therapy was for the cough symptom on day 7, with 45.7% cumulative survival vs. day 8 with 46.2%, p = 0.932; Fever day 3, 15.2% vs. day 3, 21.5%, p = 0.934; Headache day 6, 47.8% vs. day 5, 41.5%, p = 0.266; Odynophagia day 4, 43.5% vs. day 4, 40%, p = 0.641; rhinorrhea day 7, 19.6% vs. day 7, 20%, p = 0.714; Anosmia day 2, 43.5% vs. day 6, 44.6%, p = 0.209; Conjunctivitis day 1, 23.9% vs. day 1, 10.8%, p = 0.037; Diarrhea day 1, 30.4% vs. day 1, 24.6%, p = 0.116; Myalgia day 5, 45.7% vs. day 4, 43.1%, p = 0.381; Chest pain day 1, 43.5% vs. day 1, 47.7%, p = 0.491; Movement dyspnea day 1, 28.3% vs. day 1.23.1%, p = 0.750; Dyspnea at rest day 1, 17.4% vs. day 1, 20.0%, p = 0.558; respectively (Tables 6.1-6.3). In relation to adverse events, there were blood and lymphatic system disorders: 2 (4%) patients with double therapy and 2 (3%) patients with triple therapy, cardiac disorders: 4 (9%) and 5 (8%), ear and labyrinth disorders: 6 (13%) and 10 (15%), gastrointestinal disorders: 9 (20%) and 8 (12%), musculoskeletal and connective tissue disorders: 18 (39%) and 24 (37%), respectively.
Discussion
In the present study, we could consider the efficacy of Ivermectin against SARS-Cov-2 [20] given that the group of patients with triple therapy presented only 10% therapeutic failure in all patients operated on with conventional dose therapy in humans, a possible explanation This is what is referred to by a study in the experimental phase indicating that ivermectin has an anti-inflammatory effect in the inhibition of nitric oxide, prostaglandins E2 induced by the activation of macrophage lipopolysaccharides, at a dose of 0.5 and 2ug / ml [21] and in lung tissue in mice, the administration of 2mg / kg of ivermectin suppresses mucus hypersecretion, the recruitment of immune cells and the production of cytokines and IgE / IgG1 in the bronchoalveolar lavage in the lower respiratory tract. Khan, et al. [22] refers to a 1% therapeutic failure in all patients treated with ivermectin at 12 mg orally once a day during their hospital stay [23]. In the present study, the inclusion of patients with double or triple therapy was observed during the mild clinical phase of COVID-19, with initiation of therapy on the 4th day of the COVID-19 disease vs. another study including patients in a moderate clinical phase with a 9-day hospital stay [24]. In relation to Azithromycin that is within the two therapies (double and triple), only 3 (2.6%) patients died, a characteristic that was also observed in presenting the lower risk of death when using Azithromycin alone in hospitalized patients with COVID-19 , reporting an adjusted Hazard Ratio of 0.56 (95% CI, 0.26-1.21) [24]. Rivaroxaban at a dose of 15mg per day in patients with moderate phase COVID-19 increases serum levels of oral anticoagulants when treated with antiviral drugs up to 6 times more [25] in the present study, there were no serious hematological alterations in the group with and without ivermectin, given this being a prophylactic dose of rivaroxaban, there are studies in the recruitment phase that are indicating the dose of rivaroxaban at a dose of 10mg in mild and moderate phase patients with COVID-19 with remote electronic monitoring for 35 days [26].
Limitations
In the present study, the sample size was insufficient to obtain a statistically significant difference on the type of therapy implemented, however, according to the hypothesis of the study about a modification in the clinical evolution ≥ 25% of patients with COVID-19 under a treatment with double or triple therapy for 14 days followed by video call, practically in the symptoms cough, headache, odynophagia, anosmia, myalgia, chest pain and movement dyspnea, a percentage> 25% was reached in the median of patients due to symptoms in both therapies.
Conclusion
In the present study, a percentage of patients with a diagnosis of COVID-19 that modify their clinical evolution greater than 90% was achieved for both early intervention therapies, with a clinical but not statistical difference in the type of therapy, still achieving an impact on the therapeutic approach of patients with SARS-Cov2 for the improvement of health, however it is necessary to carry out more randomized clinical trials involving these therapies and to replicate these results.
Thanks
To the Head Delegate of the North Delegation who contributed to making this article a reality and to the Head of Medical Benefits who, together with the work team in charge, led to the successful completion of this research study.
Conflict of Interests
The researchers of the present information of the article declare that there is no economic interest or conflict of interest.
References
- (2020) Organización Mundial de la Salud. Coronavirus Disease (COVID-19) Outbreak.
- (2020) Instituto Mexicano del Seguro Social. COVID-19 Casos acumulados de COVID-19 en Mé [Internet]. Comisión Intersecretarial para el Desarrollo del Gobierno Electrónico, Ventanilla Única, México.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, et al. (2020) Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 382(18): 1708-1720.
- Organización Mundial de la Salud, (24 de Febrero de 2020). Report of the WHO-China Joint Mission.
- Alcocer VJ, Laurell AEC, López Gatell RH, Flores JP (2020) Lineamiento estandarizado para la vigilancia epidemiológica y por laboratorio de enfermedad respiratoria viral.
- Arshad U, Pertinez H, Box H, Tatham L, Rajoli RKR, et al. (2020) Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics. Clin Pharmacol Ther 108(4): 775-790.
- Drożdżal S, Rosik J, Lechowicz K, Machaj F, Kotfis K, et al. (2020) FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug Resist Updat 53: 100719.
- Bethesda (MD): National Library of Medicine (US) (2020) Identificador NCT04343092, Effectiveness of Ivermectin as add-on Therapy in COVID-19 Management. Clinical Trials.gov.
- Aguirre Chang, Gustavo (2020) Inclusión de la Ivermectina en la primera línea de acción terapéutica para COVID-19. Se reporta una muy significativa disminución de la Tasa de Letalidad con su uso. Research Gate.
- Lespine A, Alvinerie M, Sutra JF, Pors I, Chartier C (2005) Influence of the route of administration on efficacy and tissue distribution of ivermectin in goat. Vet Parasitol 128(3-4): 251-260.
- Choudhary R, Sharma AK (2020) Potential use of hydroxychloroquine, ivermectin and azithromycin drugs in fighting COVID-19: trends, scope and relevance. New Microbes New Infect 35: 100684.
- Dos Santos WG (2020) Natural history of COVID-19 and current knowledge on treatment therapeutic options. Biomed Pharmacother 129: 110493.
- Sargiacomo C, Sotgia F, Lisanti MP (2020) COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging (Albany NY) 12(8): 6511-6517.
- Rahman MT, Idid SZ (2020) Can Zn Be a Critical Element in COVID-19 Treatment? Biol Trace Elem Res 26: 1-9.
- Gautret P, Lagier JC, Parola P, Van Thuan Hoang, Line Meddeb, et al. (2020) Hydroxychloroquine and azithromycin as a treatment of COVID19: results of an open-label non-randomized clinical trial. Intenational Journal of Antimicrobial Agents 56(1): 105949.
- Vivas D, Roldán V, Esteve Pastor MA, Roldán I, Tello Montoliu A, et al. (2020) Revisores expertos. Recomendaciones sobre el tratamiento antitrombótico durante la pandemia COVID-19. Posicionamiento del Grupo de Trabajo de Trombosis Cardiovascular de la Sociedad Española de Cardiologí Rev Esp Cardiol 73(9): 749-757.
- Becattini C, Pace U, Rondelli F, Delrio P, Ceccarelli G, et al. (2020) Rivaroxabán for extended antithrombotic prophylaxis after laparoscopic surgery for colorectal cancer. Design of the PRO-LAPS STUDY. Eur J Intern Med 72: 53-59.
- Khorana AA, Soff GA, Kakkar AK, Vadhan Raj S, Riess H, et al. (2019) Rivaroxabán for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N Engl J Med 380(8): 720-728.
- Dugina TN, Kiseleva EV, Chistov IV, BA Umarova, SM Strukova(2002) Receptors of the PAR Family as a Link between Blood Coagulation and Inflammation. Biochemistry (Moscow) 67: 65-74.
- Heidary F, Gharebaghi R (2020) Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot (Tokyo) 73(9): 593-602.
- Zhang X, Song Y, Xiong H, Ci X, Li H, et al. (2009) Inhibitory effects of ivermectin on nitric oxide and prostaglandin E2 production in LPS-stimulated RAW 264.7 macrophages. Int Immunopharmacol 9(3): 354-359.
- Yan S, Ci X, Chen N, Chen C, Li X, et al. (2011) Anti-inflammatory effects of ivermectin in mouse model of allergic asthma. Inflamm Res 60(6): 589-596.
- Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, et al. (2020) Association of Treatment with Hydroxychloroquine or Azithromycin With In-Hospital Mortality in Patients With COVID-19 in New York State. JAMA 323(24): 2493-2502.
- Khan MSI, Khan MSI, Debnath CR, Nath PN, Mahtab MA, et al. (2020) Ivermectin Treatment May Improve the Prognosis of Patients With COVID-19. Arch Bronconeumol 56(12): 828-830.
- Testa S, Prandoni P, Paoletti O, Morandini R, Tala M, et al. (2020) Direct oral anticoagulant plasma levels' striking increase in severe COVID-19 respiratory syndrome patients treated with antiviral agents: The Cremona experience. J Thromb Haemost 18(6): 1320-1323.
- Bethesda (2020) National Library of Medicine (US). Identificador NCT04508023, A Study of Rivaroxaban to Reduce the Risk of Major Venous and Arterial Thrombotic Events, Hospitalization and Death in Medically Ill Outpatients with Acute, Symptomatic Coronavirus Disease 2019 (COVID-19) Infection (PREVENT-HD). Clinical Trials.gov.

 Research Article
Research Article