Abstract
Bone morphogenetic proteins (BMPs) are essential factors in regulating cell differentiation, proliferation, motility, and survival during embryonic, postnatal development, and tissue development. Recently aberrant expression of BMPs is implicated in multiple cancer studies suggesting a role in tumorigenesis and promoting cancer progression in different stages. Here, we have briefly summarized the significance of BMP in embryonic development and its dysfunction in cancer and the molecular regulation of BMP-related signalling pathway. The review aims to provide a brief overview of the biological and pathological importance of BMP signalling and the possibility of it being used as disease and therapy targets for better outcomes for the patients.
BMP Signalling in Early Embryonic Development
Development of a unicellular zygote to a multilayered organised
embryo, involves complex growth cues from specific growth factors,
strictly regulated cellular division and series of lineage specification
events. These two major events include the formation of a blastocyst,
at embryonic day (E) 3.5, followed by gastrulation when cells then
go on to form either the outer trophectoderm (TE) or inner cell
mass (ICM), from which the epiblast (Epi,) or primitive endoderm
(PrE,) [1] is derived (Figure 1A). First known for their ability to
induce bone formation, members of Bone Morphogenetic Proteins
(BMPSs) are now known to play crucial roles in in the regulation of
a multiple developmental processes including formation of meso
and endodermal lineages [2-4] and PGC specification [5-7] during
early embryonic development.
During gastrulation, BMP signalling acts through a receptor
complex including BMP receptor type II and ALK3/6, which results
in SMAD1/5 phosphorylation. Phosphorylated SMAD1/5 form
a complex with SMAD4 and translocate to the nucleus to control
target gene expression that direct cellular processes for PGC
specification, and formation of mesodermal lineages as evidenced in
human and mice (Figure 1B) [3,8]. Among the various BMPs, BMP4
is necessary to induce PGCs, while BMP8b an inhibitor of BMP4
signalling regulates the development of the visceral endoderm
[9,10]. Additionally, expression of BMP2 in visceral endoderm
surrounding the epiblast augments the BMP4 signal in the
posterior of the embryo. The essential functions of BMP signalling
factors is highlighted by embryonic lethality of knockout mouse
models of different components of the BMP signalling pathway.
BMP4 deficient mice lacks mesoderm and have no primordial
germ cells. BMP heterozygotes are viable but have a wide variety
of abnormalities [11] reconfirming their essential role in PGC and
essential for developmental processes as early as gastrulation.
Furthermore, BMP4 is also implicated in mouse ES (mES)
cell self- renewal and regulating fate choices during stem cell
differentiation [12]. For example, during stem cell differentiation
BMPs direct mesenchymal cells to differentiate in chondrogenic
and osteogenic cell lineages [13]. However, as in the mouse embryo,
BMP can also promote mesendodermal differentiation of mES cells
[14,15]. Additionally, BMP signalling has shown to induce inhibitory
signals to neural differentiation [16,17] and inhibiting BMP
signalling during mESC differentiation promotes differentiation of
neural ectoderm. Inhibitors of BMPs are recently characterized as
BMP antagonists. Although BMP antagonists often exert biological functions as inhibitors of BMP action, in some cases, they function
as activators of BMPs during distinct phases of development, adding
a complex feedback mechanism. In vivo experiments have verified
that this process controls intrinsic inhibitors of bone morphogenic
protein (BMP), Activin/Nodal signalling [18].
Similarly, in-vitro stem cells differentiation systems where
Noggin or the invivo BMP inhibitors are introduced, cells undergo
neuroectodermal fate [19]. On the other hand, addition of BMP
agonist promoted mesodermal and PGC specification during ES cell
differentiation. Furthermore, conditional gene targeting was used
to confirm the role of BMP signalling during PGC migration in vivo,
which demonstrated that BMP signalling controls cell survival and
possibly cell identity within the genital ridges representing an early
and probably transient PGC niche that is established in the genital
ridges before differentiation of the Sertoli or granulosa cell support
lineages [20].
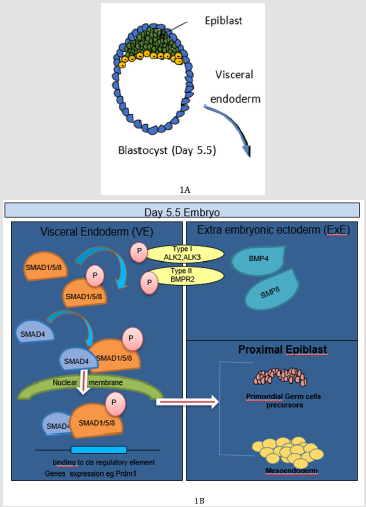
Figure 1: BMP signaling in early embryonic development. A: Representative image of Day 5.5 blastocyst. B. At Day 5.5–6.0 embryonic day BMP4 secreted by the ExE binds to BMP receptor complexes consisting of BMP receptor type II and ALK2 in the VE, which results in Smad1 phosphorylation and transcriptional regulation of BMP target genes. Among these target genes, an unknown factor (or factors) directs proximal epiblast cells toward a PGC and formation of meso-endodermal lineages.
BMPs and Their Association in Cancer
The irregular expression of BMPs is also indicated to be related to cancer cell proliferation, differentiation, and apoptosis. Specific BMPs are now used as new biomarkers for the prognosis of cancer patients [21,22]. It has been hypothesized that BMP signalling induces SMAD8 phosphorylation, which then forms a heteromeric complex with the common mediator Smad4 and is translocated into the nucleus. Inside the nucleus, the Smad8/Smad4 heterodimer itself or other Smad-interacting proteins or cofactors bind to DNA and mediate specific transcriptional activation or repression responses which may involve repression of tumour suppressor or activation of oncogenes. Possibly, there are numerous other signalling pathways such as the PI3K/Akt, P/kc, Rho-GTPases, Ras-MEK pathway, that BMP signalling has been found to interact, and could modulate their action by establishing crosstalk among the different pathways [13]. For example, the family member BMP-9 phosphorylates SMAD1, 5, and 8 and the overexpression of inhibitors of DNA binding 1 (Id1), promoting a proliferative response and exerting an anti-apoptotic function in hepatic cancer cells. BMP-9 expression is also linked to ovarian cancer. BMP-9 led to cyclinD1 protein upregulation and inhibited the expression of CDK-interacting protein p27, causing a dual effect inactivation cell proliferation and division. The BMP-downstream signalling effectors such as SMAD1, 5, and 8 are shown to promote tumours, for e.g, in hepatocellular carcinoma (HCC), high expression levels of several BMPs (BMP-4, -6, -7, -8, -9, -10, -11, -13, and -15) are related to poor prognosis [20, 23].
Similarly, in advanced non-small cell lung cancer, increased BMP- 2 level was correlated with poor prognosis. BMP-4 upregulation is also seen as shorter patients’ overall and disease-free survival and serves as a new marker for predicting cancer patients’ recurrence and prognosis after surgery. Also, high BMP-7 expression could also be a useful predictive marker of poor prognosis in patients with oesophagal squamous cell carcinoma and clear cell renal carcinoma [13]. Aside from these impacts on tumorigenesis, BMP signalling is also involved in the invasion and migration processes and promoting metastatic spread. BMPs significantly promoted tumour migration by dysregulating the extracellular matrix (ECM) environment, involving integrin and matrix metalloproteinases (MMPs), which is a crucial factor in tumour migration [23]. MP-7 upregulates integrin avb3 expression, thereby inducing the migration activity in human chondrosarcoma cells. BMPs are involved in accelerating pancreatic cancer cell invasiveness by MMP-2 upregulation. Furthermore, BMP-2-induced phosphorylation of SMAD2/3 promotes epithelialmesenchymal transition (EMT) and shown to induce cell invasion and migration in breast and pancreatic cancer cells [24].
Like Noggin, Gremlin [4] is a BMP antagonist. Gremlin 1 knockdown suppresses cancer stem cell (CSC) proliferation and tumour development in various cancer models. This role of Gremlin 1 is thought to be highly correlated with inciting cell cycle progression in CSCs via p21 [25]. Gremlin 1 knockdown suppresses cancer stem cell (CSC) proliferation and tumor development in various cancer models. This function of Gremlin 1 is believed to be highly associated with stimulating cell cycle progression in CSCs via p21 [26]. Similarly, Noggin’s overexpression leads to decreased tumour size and reduced bone loss compared to control animals in prostate cancer (PC) cells implanted with tibias [26]. Moreover, BMPs are considered multifunctional cytokines belonging to the TGF-β superfamily. Like other members of the TGF-β superfamily, BMPs can bind and form heteromeric complexes with two types of serine/threonine kinase receptors (type I and type II) on the cell surface, both of which are required for signal transduction [26,27].
The role of BMPs in tumour progression and dissemination
has been widely published in previous studies. However, some
studies revealed an opposite role of BMP signalling in tumors. BMP-
10 [28-30] seems to be downregulated in gastric cancer samples.
BMP-6 expression was absent in breast cancer tissues and might
suppress breast cancer metastasis. Inhibition of BMP signalling
significantly downregulates protein levels of multiple mitotic
checkpoint components, leading to increased cell division and
tumourigenesis, whereas an upregulation of BMP signalling has the
reverse effect in breast cancer cells. For instance, BMP-2 and BMP-7
function as tumor suppressors in multiple solid tumours, where it
suppresses tumor growth by reducing the expression of oncogenic
factors and promoting the expression of cell differentiation inducer
proteins [31]. In hepatocellular carcinoma (HCC), BMP-2 induces
the expression of the pro-apoptotic protein’s caspase-3 and cleaved
caspase-3 leading to increased apoptosis and plays an anti-cancer
protein [31-34].
Similarly, In HCC, the inhibition of the BMP-4/SMAD1 signaling
has been reported to suppress tumor migration, invasion, and EMT
[35]. Moreover, BMP-4 and BMP-9 were also found to be potential
anticancer agents in many cancer. Expression of BMP-4 reduces the
number and activities of myeloid-derived suppressor cells (MDSCs)
by declining the secretion of granulocyte colony-stimulating factor
(G-CSF). BMP-9 is also shown to inhibit bone metastasis of breast
cancer cells by downregulating connective tissue growth factor
expression [26]. It induces apoptosis in prostate cancer cells’
growth, which is related to the upregulation of prostate apoptosis
response 4. In many solid tumors, BMP-4 paraclinically inhibits
tumor angiogenesis via the induction of thrombospondin-1 (TSP-
1). Sometimes, SMADs in the BMP signaling pathway are also
linked to tumor suppression. Knocking down SMAD1 and SMAD5
promotes metastatic granulosa cell tumorigenesis in somatic cells
of male and female gonads of mice, which implicated SMAD1 and
SMAD5 as critical tumor suppressors [36].
In conclusion, BMPs are described as both stimulator and
inhibitor in different cancers; thus, we cannot simply outline BMPs as oncogenes or anti-oncogenes. Together, the aforementioned
evidence indicated that the effects of BMP signaling on tumor
progress depends on the cell types and the tumor microenvironment.
Therefore, it’s important to analyse the BMP bilateral effects in
tumorigenesis and the underlying signaling pathways regulating
the enigmatic dilemmas rom the translational medicine perspective.
Such analysis may also affect the choice of specific BMP inhibitors,
with varying selectivity, should these agents become available in
the clinic.
References
- Yamamoto Y, M Oelgeschlager (2004) Regulation of bone morphogenetic proteins in early embryonic development. Naturwissenschaften 91(11): 519-534.
- Davis S, Miura S, Hill C, Mishina Y, Klingensmith J (2004) BMP receptor IA is required in the mammalian embryo for endodermal morphogenesis and ectodermal patterning. Dev Biol 270(1): 47-63.
- Graham SJ, Krzysztof B Wicher, Jedrusik A, Guo G, Herath W (2014) BMP signalling regulates the pre-impla ntation development of extra-embryonic cell lineages in the mouse embryo. Nat Commun 5: 5667.
- Manisastry SM, M Han, KK Linask (2006) Early temporal-specific responses and differential sensitivity to lithium and Wnt-3A exposure during heart development. Dev Dyn 235(8): 2160-2174.
- Panula S, Medrano JV, Kee K, Bergstrom R, Nguyen HN, et al. (2011) Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum Mol Genet 20(4): 752-762.
- Sato T, J Ogata, Y Niki (2010) BMP and Hh signaling affects primordial germ cell division in Drosophila. Zoolog Sci 27(10): 804-810.
- Senft AD, Elizabeth K Bikoff, Elizabeth J Robertson, Ita Costello (2019) Genetic dissection of Nodal and Bmp signalling requirements during primordial germ cell development in mouse. Nat Commun 10(1): 1089.
- Soares ML, Haraguchi S, Torres Padilla ME, Kalmar T, Carpenter L, et al. (2005) Functional studies of signaling pathways in peri-implantation development of the mouse embryo by RNAi. BMC Dev Biol 5: 28.
- Bosman EA, Lawson KA, Debruyn J, Beek L, Francis A, et al. (2006) Smad5 determines murine amnion fate through the control of bone morphogenetic protein expression and signalling levels. Development 133(17): 3399-3409.
- Shirazi R, Zarnani AH, Soleimani M, Abdolvahabi MA, Nayernia K, et al. (2012) BMP4 can generate primordial germ cells from bone-marrow-derived pluripotent stem cells. Cell Biol Int 36(12): 1185-1193.
- Chen D, M Zhao, GR Mundy (2004) Bone morphogenetic proteins. Growth Factors 22(4): 233-241.
- Zhao GQ (2003) Consequences of knocking out BMP signaling in the mouse. Genesis 35(1): 43-56.
- Wang RN, Green J, Wang Z, Deng Y, Qiao M, et al. (2014) Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis 1(1): 87-105.
- Zhang WV. NS Stott (2004) BMP-2-modulated chondrogenic differentiation in vitro involves down-regulation of membrane-bound beta-catenin. Cell Commun Adhes 11(2-4): 89-102.
- Yuan G, Yang G, Zheng Y, Zhu X, Chen Z, et al. (2015) The non-canonical BMP and Wnt/beta-catenin signaling pathways orchestrate early tooth development. Development 142(1): 128-139.
- Zhu XJ, Liu Y, Yuan X, Wang M, Zhao W, et al. (2016) Ectodermal Wnt controls nasal pit morphogenesis through modulation of the BMP/FGF/JNK signaling axis. Dev Dyn 245(3): 414-426.
- Zhang K, Li L, Huang C, Shen C, Tan F, et al. (2010) Distinct functions of BMP4 during different stages of mouse ES cell neural commitment. Development 137(13): 2095-2105.
- Xu X, VL Browning, JS Odorico (2011) Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech Dev 128(7-10): 412-427.
- Smith KA, Noel E, Thurlings I, Rehmann H, Chocron S, et al. (2011) Bmp and nodal independently regulate lefty1 expression to maintain unilateral nodal activity during left-right axis specification in zebrafish. PLoS Genet 7(9): e1002289.
- Yoshioka Y, Ono M, Osaki M, Konishi I, Sakaguchi S (2012) Differential effects of inhibition of bone morphogenic protein (BMP) signalling on T-cell activation and differentiation. Eur J Immunol 42(3): 749-759.
- Hu X, Zhang L, Li Y, Ma X, Dai W, et al. (2020) Organoid modelling identifies that DACH1 functions as a tumour promoter in colorectal cancer by modulating BMP signalling. EBio Medicine 56: 102800.
- Blanco Calvo M, Bolos Fernandez V, Medina Villaamil V, Aparicio Gallego G, Diaz Prado S, et al. (2009) Biology of BMP signalling and cancer. Clin Transl Oncol 11(3): 126-137.
- Zhang L, Ye Y, Long X, Xiao P, Ren X, et al. (2016) BMP signaling and its paradoxical effects in tumorigenesis and dissemination. Oncotarget 7(47): 78206-78218.
- Bayramov AV, Fedor M Eroshkin, Natalia Y Martynova, Galina V Ermakova, Elena A Solovieva, et al. (2011) Novel functions of Noggin proteins: inhibition of Activin/Nodal and Wnt signaling. Development 138(24): 5345-5356.
- Chen B, Athanasiou M, Gu Q, Blair DG (2002) Drm/Gremlin transcriptionally activates p21(Cip1) via a novel mechanism and inhibits neoplastic transformation. Biochem Biophys Res Commun 295(5): 1135-1141.
- Bach DH, HJ Park, SK Lee (2018) The Dual Role of Bone Morphogenetic Proteins in Cancer. Mol Ther Oncolytics 8: 1-13.
- Giacomini D, Acuna M, Gerez J, Nagashima AC, Silberstein S, et al. (2007) Pituitary action of cytokines: focus on BMP-4 and gp130 family. Neuroendocrinology 85(2): 94-100.
- Suzuki Y, Ohga N, Morishita Y, Hida K, Miyazono K, et al. (2010) BMP-9 induces proliferation of multiple types of endothelial cells in vitro and in vivo. J Cell Sci 123(Pt 10): 1684-1692.
- Bessa PC (2009) Expression, purification and osteogenic bioactivity of recombinant human BMP-4, -9, -10, -11 and -14. Protein Expr Purif 63(2): 89-94.
- Ali AA, Mukhtar MM, Shaheen S, Mohamed AO (2021) Assessment of plasma BMP-2, BMP-7, BMP-10, vitamin D, and TGF beta1 in simple fractures among Sudanese patients. PLoS One 16(2): e0247472.
- Sun Z, Cai S, Zabkiewicz C, Liu C, Ye L (2020) Bone morphogenetic proteins mediate crosstalk between cancer cells and the tumour microenvironment at primary tumours and metastases (Review). Int J Oncol 56(6): 1335-1351.
- Zoheiry MM, Aa Hasan S, El-Ahwany E, Nagy FM, Taleb HA, et al. (2015) Serum Markers of Epithelial Mesenchymal Transition as Predictors of HCV-induced Liver Fibrosis, Cirrhosis and Hepatocellular Carcinoma. Electron Physician 7(8): 1626-1637.
- Ye L, WG Jiang (2016) Bone morphogenetic proteins in tumour associated angiogenesis and implication in cancer therapies. Cancer Lett 380(2): 586-597.
- Shikauchi Y, Saiura A, Kubo T, Niwa Y, Yamamoto J, et al. (2009) SALL3 interacts with DNMT3A and shows the ability to inhibit CpG island methylation in hepatocellular carcinoma. Mol Cell Biol 29(7): 1944-1958.
- Wang Y, Sun B, Zhao X, Zhao N, Sun R, et al. (2016) Twist1-related miR-26b-5p suppresses epithelial-mesenchymal transition, migration and invasion by targeting SMAD1 in hepatocellular carcinoma. Oncotarget 7(17): 24383-24401.
- Pangas SA, Li X, Umans L, Zwijsen A, Huylebroeck D, et al. (2008) Conditional deletion of Smad1 and Smad5 in somatic cells of male and female gonads leads to metastatic tumor development in mice. Mol Cell Biol 28(1): 248-257.

 Mini Review
Mini Review