Abstract
The present work described the development of a high-performance liquid chromatographic (HPLC) method for determination atomoxetine & fluoxetine in their pharmaceutical dosage forms Strattera® & Prozac®. The analysis was achieved on Thermo Hypersil BDS C18 column (250mm x 4.6mm i.d, 5m particle size) using mixture of 375ml of distilled water containing 0.1ml tetra-n-butylammonium hydroxide + 0.4ml triethylamine (adjust pH to 3.5 with phosphoric acid) & 625ml Acetonitrile (375: 625, v/v) as mobile phase at flow rate of 1ml/min and UV detection at 220nm. The developed method was linear over the concentration range of 1-16g/ml (r = 0.99999) with a limit of detection 0.028 and 0.065g/ml for atomoxetine & fluoxetine respectively and a limit of quantitation 0.085 and 0.198g/ml for atomoxetine & fluoxetine respectively. The developed HPLC method was validated with respect to specificity, linearity, accuracy, precision, limit of detection and limit of quantitation. The statistical analysis proved that the developed method for quantification of atomoxetine & fluoxetine as bulk drug and from pharmaceutical preparation is reproducible and selective. The proposed HPLC method can be used for the quality control of formulated products containing atomoxetine or fluoxetine or both.
Keywords: (HPLC); Simultaneous; Atomoxetine HCl; Fluoxetine HCl
Introduction
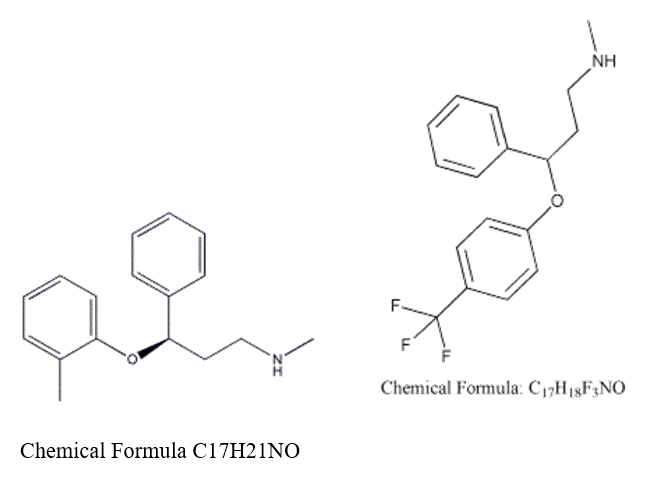
Atomoxetine HCl
Atomoxetine is the first non-stimulant drug approved for the treatment of an attention-deficit hyperactivity disorder (ADHD). It is sold in the form of Hydrochloride salt of Atomoxetine. It is a selective nor-adrenaline inhibitor. The formal chemical name (IUPAC) is (R)-N-methyl-3-phenyl-3-(o-tolyloxy) propan-1-amine (Figure 1) [1]. Atomoxetine is classified as a norepinephrine reuptake inhibitor, and is approved for use in children, adolescents, and adults. However, its efficacy has not been studied in children under six years old. Its advantage over stimulants for the treatment of ADHD is that it has less abuse potential than stimulants, is not scheduled as a controlled substance and has proven in clinical trials to offer 24-hour coverage of symptoms associated with ADHD in adults and children [1]. Strattera was originally intended to be a new antidepressant drug; however, in clinical trials, no such benefits could be proven. Since norepinephrine is believed to play a role in ADHD, Strattera was tested and subsequently approved as an ADHD treatment. Clinical experiments are currently being undertaken to test Atomoxetine use in weight loss programs with obese people or those with a binge eating disorder [2].
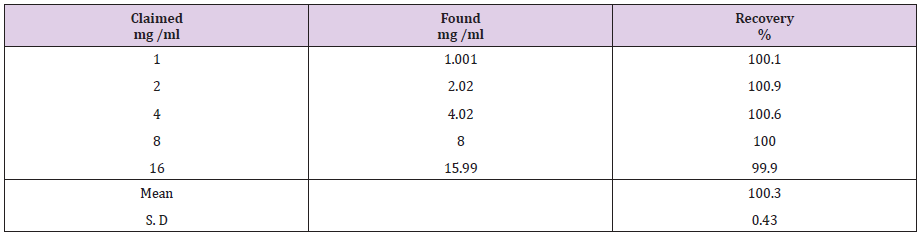
Fluoxetine HCl
Fluoxetine HCl is a selective serotonin reuptake inhibitor, antidepressant. (3RS)-N-Methyl-3-phenyl-3-[4-trifluoromethyl) phenoxy] propan-1-amine hydrochloride [3]. Fluoxetine is used in treatment of depression in both adults and children aged 8 years and over and in the management of bulimia nervosa, obsessive - compulsive disorder, it may be used in panic disorder and in the treatment of pre-menstrual dysphoric disorder [4]. Literature survey reveals that several methods have been reported like UV , HPLC, HPLC-MS, HPTLC for the assay of atomoxetine HCL [5-20] and also several methods have been reported for the assay of fluoxetine HCL [21-31] but as far as we aware there is only one HPLC method for them together [18] the proposed method is supposed to be very rapid and most simple to achieve good estimation of the concentrations of atomoxetine HCl and fluoxetine HCl together without any interfere, and with minimum cost.
Experimental
Apparatus
a) Agilent HPLC system
b) Cole Parmer pH meter
Materials and Reagents
Acetonitrile was of HPLC grade, phosphoric acid, tetra-nbutylammonium
hydroxide and triethylamine were analytical
grades.
a) Authentic: atomoxetine HCl, kindly supplied by Lilly.
Fluoxetine HCl, kindly supplied by Lilly.
b) Pharmaceutical Formulation: Strattera ®, Prozac ®
HPLC Conditions
The HPLC separation and quantitation were achieved on:
a) Column: Thermo BDS Hypersil C18 column (250mm x
4.6mm i.d, 5μm particle size)
b) Mobile Phase: 375ml of distilled water containing 0.1ml
tetra-n-butylammonium hydroxide + 0.4ml triethylamine (adjust
pH to 3.5 with phosphoric acid) & 625ml Acetonitril (375: 625, v/v)
c) Flow Rate: 1ml/min
d) Injection Volume: 20μl
e) Temperature: ambient temperature
f) Detector: 220nm
Standard Solution
Stock solution of atomoxetine (1mg/ml) was prepared by dissolving a weight of atomoxetine HCL which is equivalent to 100 mg of atomoxetine base in 100ml of mobile phase. Stock solution of fluoxetine (1mg/ml) was prepared by dissolving a weight of fluoxetine HCl which is equivalent to 100 mg of fluoxetine base in 100ml of mobile phase.
Preparation of Calibration Curve
The working solution was prepared by further dilution of the stock standard solution with the mobile phase to reach the concentration range of 1-16μg/ml for both atomoxetine & fluoxetine. Triplicate 20μl injections were made for each concentration and chromatographed under the specified chromatographic conditions described previously. The peak area values were plotted against corresponding concentrations, linear relationship was obtained.
Pharmaceutical formulation preparation
The content of ten capsules of both Strattera® & Prozac® were weighed. A portion of the powder equivalent to 40mg of atomoxetine & fluoxetine was accurately weighed, transferred separately to 100ml volumetric flask and dissolved in 100ml mobile phase using ultrasonic bath (15 min) and then filtered through 0.45μm membrane filters (Millipore, Milford, MA). Further dilution was carried out with mobile phase to reach calibration range.
System Suitability
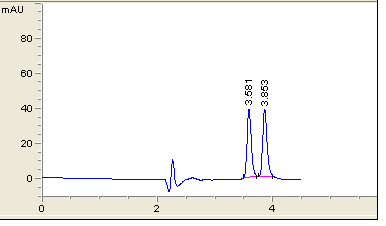
The system suitability parameters including capacity factor (k′), selectivity (α), resolution (Rs), tailing factor (T) and theoretical plate (N) listed in Table 1. All parameters were satisfactory with good specificity for the stability assessment of atomoxetine & fluoxetine.
Application to Pharmaceutical Formulation
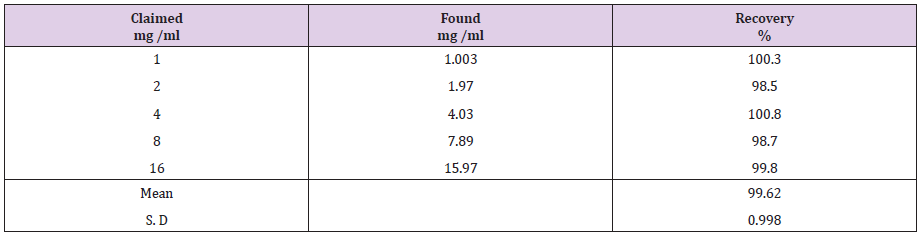
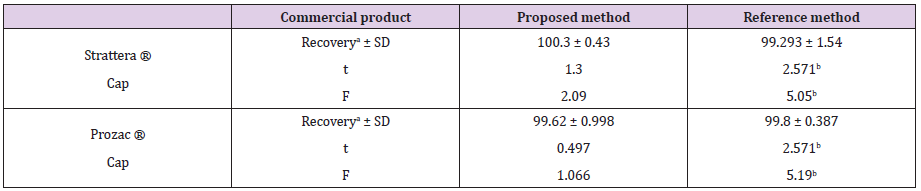
The proposed method was successfully applied to determine atomoxetine and fluoxetine in their dosage forms (Strattera® and Prozac®) capsules. Five replicates determination were made. Satisfactory results were obtained for atomoxetine and fluoxetine in good agreement with label claims (Table 2). The results obtained were compared statistically by Student′s t - test (for accuracy) and variance ratio F - test (for precision) with the reported methods [32,33]. The results in Table 3 showed that the t and F values were smaller than the tabulated values indicating that there was no significant difference between the proposed and reported methods.
Results and Discussion
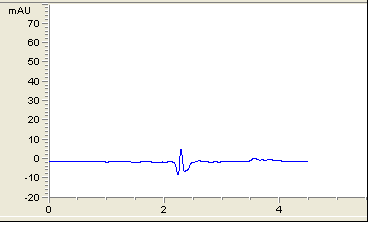
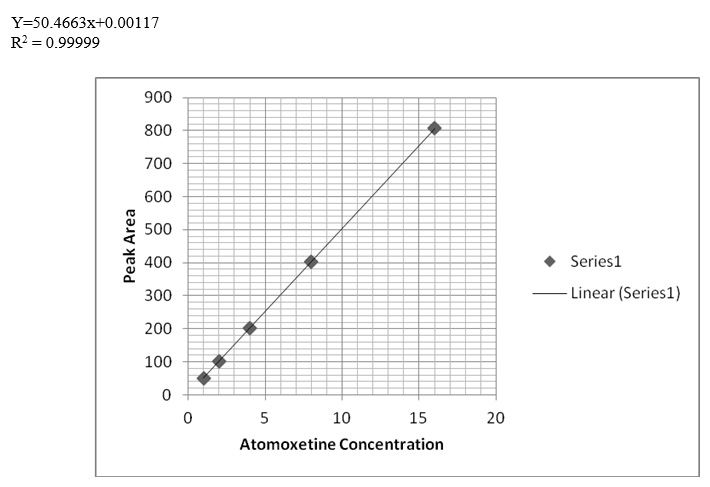
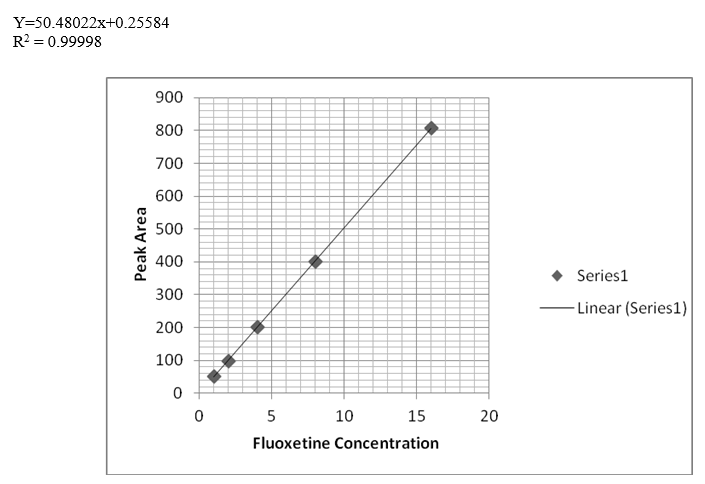
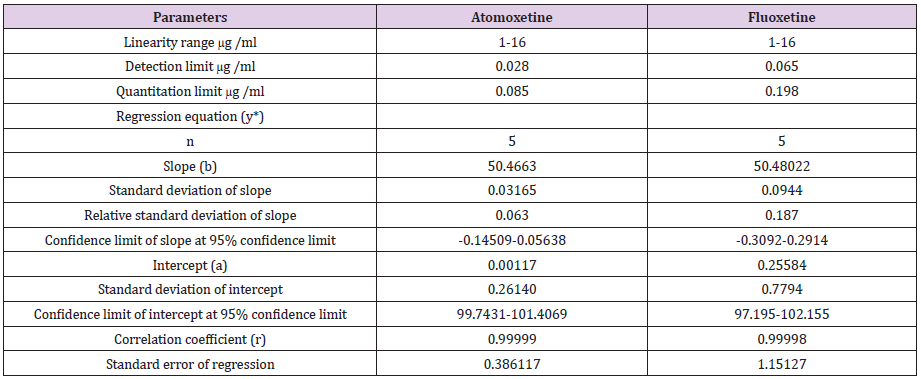
After the trials of various mobile phase systems such as 0.1% phosphoric acid solution: Acetonitrile (35:65 v/v), acetonitrile: sodium hexane sulfonate pH4 (70:30 v/v), methanol: phosphate buffer pH3.5 : THF ( 30:63:7 v/v/v), the proposed mobile phase which is a solution of tetra-n-butylammonium hydroxide + triethylamine (adjust pH to 3.5 with phosphoric acid): Acetonitrile (375 : 625, v/v) was found to be satisfactory giving good resolution peaks also several columns and PHs were tested to achieve the best resolution Figure 2. Calibration curve of atomoxetine HCl and fluoxetine HCl were prepared by plotting graphs of concentrations v/s areas (Figures 3-5) and equations for straight line was obtained.
Validation of the Method
Linearity
The linearity of the proposed method for determination of atomoxetine and fluoxetine was validated by analyzing different concentrations of the drugs. According to the international conference on harmonization ICH [34], at least five concentrations must be used. In this study five concentrations were chosen, ranging between 1-16 μg/ml of atomoxetine and fluoxetine. Each concentration was repeated three times. The high value of correlation coefficient and the intercept value was not statistically (p<0.05) different from zero (Table 4) validate the linearity of the calibration graphs. Typically, the regression equation was Y= 0.0117 + 50.4663 C (r = 0.99999) for atomoxetine and Y= 0.25584 + 50.48022 C (r = 0.99998) for fluoxetine
Table 4: Determination of fluoxetine in Prozac ® capsules by the proposed HPLC method.
a: Mean of six determinations. b: The theoretical values of t and F at P = 0.05.
Precision
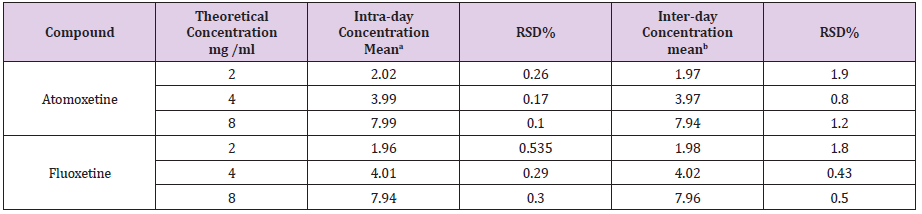
To prove the validity and applicability of the proposed method and reproducibility of the results mentioned, three concentrations of the cited drugs were carried out. Table 5 show the values of Intraday and Inter-day relative standard deviation (RSD %) for different concentrations.
Range
The calibration range was established through consideration of the practical range necessary, according to the drug concentration present in the pharmaceutical product, to give accurate, precise and linear results. The calibration range of the proposed method is given in Table 4.
Table 5: Assay parameters and regression characteristic of atomoxetine determined by the proposed HPLC method.
Y = a + b C, where C is the concentration of compound in g /ml and Y is the peak area.
Limit of Detection and Quantitation
According to ICH recommendation [34] the approach based on
the S.D of the response and the slope was used for determination
of the detection (DL) and quantitation limit (QL) by means of the
following equations:
DL = 3.3 σ S
Where σ = the standard deviation of the response
S = the slope of the calibration curve
QL = 10 σ S
Where σ = the standard deviation of the response
S = the slope of the calibration curve
Specificity
Peak purity was examined using photodiode array detector and indicate specificity of the method.
Accuracy
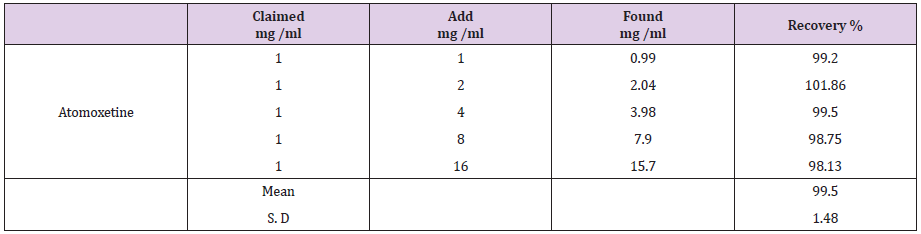
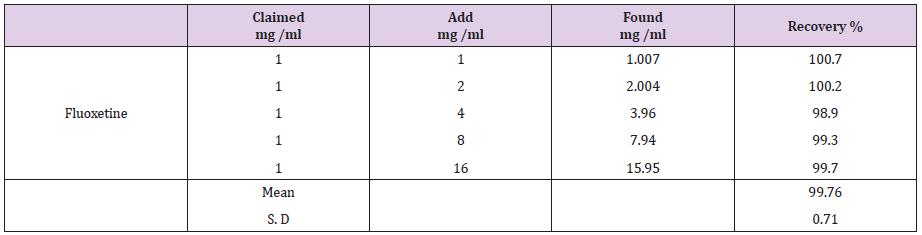
This study was performed by adding known amounts of the studied drugs compound to known concentration of the commercial pharmaceutical capsules (standard addition method). The resulting mixtures were analyzed and the results obtained were compared with the expected results (Table 6) suggested the good accuracy of the proposed methods.
Table 6: Evaluation of precision for the determination of atomoxetine by the proposed HPLC method.
aMean of three determinations.
bMean of three different days.
Robustness
Robustness is the measure of capacity of analytical methods to remain unaffected by small but deliberating variations of the operation parameters. Variation of the pH of (aqueous triethylamine and tetra-n-butylammonium hydroxide) of the mobile phase by ± 0.2, organic solvent strength of the mobile phase by ± 2 % and detector wavelength by ± 2nm did not have significant effect on chromatographic resolution of the HPLC method.
Solution stability
Solution of studied drug in mobile phase exhibit no absorbance or chromatographic change for 2weeks when kept at room temperature, and for 3 months when stored in the refrigerator at 4°C (Tables 7&8).
Table 7: The application of Standard addition technique to the analysis of atomoxtine by the proposed HPLC method.
Table 8: The application of Standard addition technique to the analysis of fluoxetine by the proposed HPLC method.
Conclusion
The proposed HPLC method provide simple, accurate, and reproducible quantitative analysis for determination of atomoxetine and fluoxetine in bulk and in pharmaceutical dosage forms without any interference from the excipients. The method was completely validated. Owing to its sensitivity, simplicity and short analysis time, it is suitable for routine analysis in quality control laboratories to assay the two drugs.
References
- https://en.wikipedia.org/wiki/Atomoxetine.
- https://www.3dchem.com/Atomoxetine.asp
- British Pharmacopoeia pp. 889.
- Martindale.
- Authorized USP pending monographs guideline.
- (2012) Der Pharma Chemica 4(1): 194-201.
- (2007) G Wei, L Wenbiao, G Guixin, Z Jun, J Chrom B 854: 128-134.
- (2008) F Peter, A bernard, J Pharm Biomed Anal 46: 431-441.
- (2005) H John, L Richard, D George, J. Pharm. Biomed. Anal 38: 720-733
- (2011) PN Raveshiya, HR Prajapati, J Pharm Res 4(6):1720-1722.
- (2007) J-Chromatogr-B: -Anal- Technol-Biomed-Life-Sci 846(1): 351-354.
- (2005) J-pharm-biomed-Anal 38(4): 720-733.
- (2006) J-pharm-Biomed- Anal 41(4): 1088-1094.
- Chaula Patel, Minal Patel, Shubha Rani, Manish Nivsarkar, Harish Padh Journal of Chromatography B 850(1-2): 356-360.
- M Pérez-Ortiz, C Muñoz, C Zapata-Urzúa, A Álvarez-Luej Talanta 82(1): 398-403.
- (2012) Der Pharma Chemica 4(1): 194-201.
- (2010) J AOAC Int 93(4): 1207-1214.
- (2008) Journal -of- liquid- chromatography -and -related- technologies 31(5-8): 722-732.
- (2008) chromatographia Journal 67(1-2): 143-146.
- (2007) Journal- of- chromatography, B: - Analytical technologies -in -the- Biomedical- and -life-sciences 854(1-2): 128-134.
- British Pharmacopoeia. Ph Eur monograph 1104 pp. 889.
- (USP 32).
- (2008) J-Liq-Chromatogr-Relat-Technol 31(5-8): 722-732.
- (2007) J-Anal-Toxicol 31(6): 334-341.
- (2007) Chromatographia 66(1-2): 111-114.
- (2007) J-Chromtogr -B: -Anal-Technol-Biomed-Life-Sci 852(1-2): 519-528.
- (2007) J-Chromtogr B: -Anal-Technol-Biomed-Life-Sci 847(2): 217-223.
- (2006) J-Chromtogr B: -Anal-Technol-Biomed-Life-Sci 843(1): 100-113.
- (2005) Talanta. Apr 66(3): 659-663.
- (2005) J-Chromtogr, B: -Anal-Technol-Biomed-Life-Sci. 820(1): 33-39.
- (2005) Ther-Drug-Monit 27(1): 38-43.
- (2011) USP 34 NF 29 2: 2877-2878
- Straterra ® registeration file provided by Lilly.
- International conference on Harmonization (ICH).

 Research Article
Research Article