Abstract
Objective: Aimed to identify clinical features of coronavirus disease 2019 (COVID-19) in children and evaluate the role of procalcitonin in early differential diagnosis.
Methods: A retrospective analysis was performed on all suspected pediatric cases. Children are defined as being less than 18 years old. Real-time reverse transcriptase polymerase chain reaction was used to detect severe acute respiratory syndrome coronavirus 2 positive in nasopharyngeal specimens to confirm diagnosis.
Results: 39 (50.6%) of 77 suspected cases were confirmed, 4 (5.2%) of them had viral co-infection. Compared to the non-COVID-19 group (n=33), the COVID-19 confirmed group (n=39) had fewer cases of fever (Odds ratio [95% Confidence interval] 0.467[0.314- 0.694];P=.000) and symptoms (0.533[0.36–0.788];P=.001), were more asymptomatic (13.568[1.895-96.729];P=.000), and had more family cluster infections (5.077[2.224- 11.591];P=.000), while computed tomography had more positive findings (1.822[1.143- 2.906];P=.008). Age and gender were statistically insignificant. Procalcitonin of the COVID-19 alone (n=35) group had significant differences (0.05[0.029-0.076] vs 0.103[0.053-0.21];P=.000 and 0.144[0.109-2.26]; P=.010) compared with that of the non-COVID-19 and co-infection (n=4) groups. The area under curve (AUC) of model is 0.834 ([95% CI][0.741-0.926];P=.000). Procalcitonin as a differential indicator of COVID-19 alone; its AUC is 0.809 ([0.710-0.909];P=.000). The optimal cut-off value is 0.1 ng/mL, the sensitivity, specificity, positive and negative predictive value of differentiating are 65.9%, 78.6%, 82.9%, and 59.2%, respectively.
Conclusions: Asymptomatic or mild symptoms, positive computed tomography findings and family cluster infection are the main clinical features of COVID-19 in children. With good performance, procalcitonin can provide an important basis for differentiating COVID-19 alone and other viral infections or viral co-infections.
Keywords: Children; Coronavirus Disease 2019; Pneumonia; Procalcitonin; Biomarker
Abbreviations:ARI: Acute Respiratory Infection; AUC: Area Under Curve; CI: Confidence Interval; COVID-19: Novel Coronavirus Disease 2019; CT: Computed Tomography; hs-CRP: High Sensitivity C-Reactive Protein; Hb: Hemoglobin; INFA,B: Influenza A,B; IQR: Interquartile Range; LC: Lymphocyte Count; L%: Percentage Of Lymphocyte; N%: Percentage of Neutrophil; NC: Neutrophil Count; OR: Odds Ratio; PCT: Procalcitonin; PLT: Platelet; ROC: Receiver Operating Characteristic Curve; RSV: Respiratory Syncytial Virus; RT-PCR: Real-Time Reverse Transcriptase Polymerase Chain Reaction; SD: Standard Deviation; SE: Standard Error; WBC: White Blood Cell
Introduction
Children are naturally susceptible to various respiratory
viruses due to their immature immune systems. Since the outbreak
of coronavirus disease 2019 (COVID-19) began in Wuhan city,
Hubei province, China [1,2], more than 2,000 pediatric cases have
been reported nationwide in just over two months [3]. Limited
by accuracy of real-time reverse transcriptase polymerase chain
reaction (RT-PCR) detection [4], relative reagent shortage and
non-specificity of imaging findings, early differential diagnosis
of suspected pediatric patients is difficult to some extent [5].
Obviously, this has a serious impact on timely triage and the
following reasonable treatment.
At present, a growing number of studies have focused on
diagnosis and treatment of confirmed cases [6-8], but few data
are available on clinical characteristics and early identification of suspected pediatric patients with COVID-19 as a special population.
As a traditional biomarker, procalcitonin (PCT) has shown superior
value in differentiating bacterial and viral infections as well as
bacterial co-infections. However, the role of PCT in identifying
between viruses and viruses or viral co-infection remains unknown.
We aimed to identify the clinical features of COVID-19 in children
and evaluate the role of PCT in early differential diagnosis, so as to
provide a basis for the following timely and reasonable treatment
and effective prevention and control of COVID-19.
Materials and Methods
The studies involving human participants were reviewed and approved by the Ethics Committee of The Third People’s Hospital of Shenzhen (approval number:2020-123). Written informed consent from the patients was not required to participate in this study in accordance with the national legislation and the institutional requirements. Patient’s personal information will be strictly protected.
Definition and Classification
Children are defined as being less than 18 years old. We followed
the guidelines on the diagnosis and treatment of 2019 novel
coronavirus infected pneumonia (the sixth edition draft) issued by
the National Health Commission of China [9]. Suspected cases are
defined as having a clear epidemiological exposure, with or without
clinical manifestations, and with or without positive computed
tomography (CT) findings. Epidemiological exposure includes close
contact with confirmed cases, and or living or traveling in endemic
areas (especially Hubei province), and or presence of confirmed
case in their residential communities. If nasopharyngeal swab
specimens tested positive for severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) using RT-PCR detection, a suspected
case is identified as a confirmed case. Fever was recognized when
body temperature is higher than or equal to 37.3 ℃. Symptoms
of acute respiratory infection (ARI) includes nasal congestion,
runny nose, sneezing, sore throat, cough, expectoration, chest pain,
dyspnea. All chest CT images were reviewed by two experienced
pediatric radiologists. If unilateral or bilateral lung fields have any
of the features as follows:
(a) Ground glass opacities,
(b) Consolidations with surrounding halo sign,
(c) Nodules,
(d) Fibrous cord or linear opacities,
(e) Lymphadenopathy, the result is defined as positive CT
findings of viral pneumonia [10].
Family cluster infection is defined as the occurrence of any of
the following criteria in 2 or more family members within a period
of less than 1 week:
(a) Fever,
(b) Symptoms of ARI,
(c) Positive CT findings of viral pneumonia.
Data Collection and Review
For all suspected pediatric cases, we retrieved electronic
medical records and conducted a retrospective study on all
clinical and laboratory data. From Jan 22nd to Mar 1st, 2020, all
suspected pediatric patients were admitted to the Third People’s
Hospital of Shenzhen, and relevant examinations were completed
as routine procedures, and clinical and laboratory data of the first
day after admission were collected. Based on laboratory pathogen
identification results including 2019 novel coronavirus, influenza
A, B (INF A, INF B), respiratory syncytial virus (RSV), mycoplasma
pneumoniae (MP), and bacteria, all suspected cases were divided
into the COVID-19 confirmed group and the non-COVID-19 group.
The COVID-19 confirmed group was further divided into COVID-19
alone and co-infection groups, and the differences between the
groups were compared.
Inclusion criteria: all suspected pediatric cases. Exclusion
criteria of the non-COVID-19 group:
(a) Pathogen identified as bacteria or MP,
(b) Co-infection,
(c) PCT > 0.5 ng/mL [11].
Statistical Analysis
All analyses were conducted by using of IBM Statistical Product and Service Solutions software Version 24 (SPSS Inc, Chicago, IL). Continuous variables were summarized as the median with their interquartile ranges (IQRs) or mean with standard deviations (SDs), median [IQR] or [mean ±SD], depending on whether their distributions were normal or not. Comparisons of categorical variables were performed using the Pearson Chi‑square test. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for statistically significant variables. The parametric tests (independent sample Student t-test or One-way analysis of variance) or nonparametric tests (Mann-Whitney U test or Kruskal-Wallis test) were used to analyze variables. The P value was adjusted by the Bonferroni method for comparison between groups. Variables with P < 0.2 in the laboratory data analysis were entered into a multivariate binary logistic regression model. Model fitness was assessed with the Hosmer-Lemeshow goodness-of-fit test. Analysis of the area under curve (AUC) of receiver operating characteristic curve (ROC) was constructed to assess the differentiating performance. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) were also determined. P <.05 was considered as statistically significant in all tests if applied.
Results
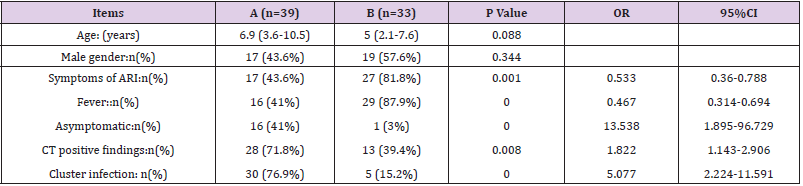
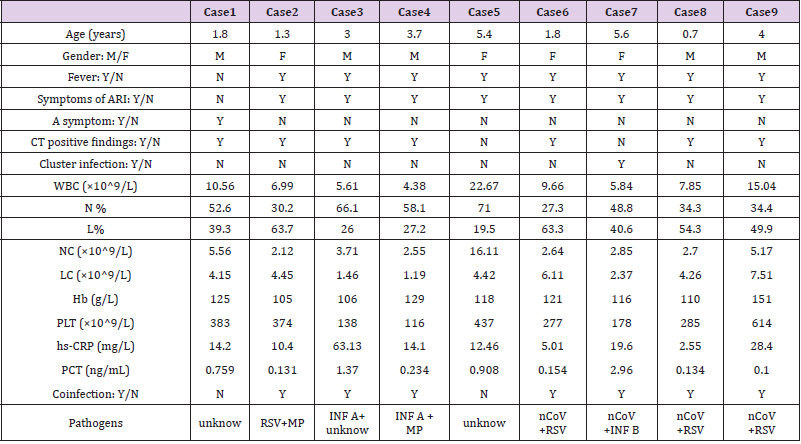
From Jan 22nd to Mar 1st, 2020, a total of 77 suspected pediatric patients were admitted, and 39 (50.6%) were confirmed with COVID-19, including 3 (3.9%) cases of RSV co-infection, and 1 (1.3%) case with INF B co-infection. 5 (6.5%) of 38 (49.4%) cases of non-COVID-19 were excluded by laboratory results and CT findings; 3 (3.9%) cases with PCT greater than 0.5 ng/mL, were considered as bacterial infection, and 2 (2.6%) cases were considered as MP co-infection (Supplement Table 1). The included 33 (42.9%) patients consisted of 3 (3.9%) cases of INF A, 2 (2.6%) cases of INF B, 9 (11.7%) cases of RSV and 19 (18.2%) cases of unidentified non-bacterial pathogens. Compared to the non-COVID-19 group (n=33), the COVID-19 confirmed group (n=39) had fewer cases of fever (OR[95%CI]0.467[0.314-0.694];P=.000) and symptoms of ARI (0.533[0.36–0.788];P=.001), were more asymptomatic (13.568[1.895-96.729];P=.000), and had more family cluster infections (5.077[2.224-11.591];P=.000), while the chest CT had more positive findings of viral pneumonia (1.822[1.143- 2.906];P=.008). Age (6.9[3.6-10.5] vs 5[2.1-7.6];P=.088) and gender (43.6% vs 57.6%;P=.344) were statistically insignificant (Table 1). Among all of four co-infection cases, four had fever and symptoms of ARI, three had positive CT findings and one was family cluster infection (Supplement Table 1).
Table 1: Clinical data analysis between the COVID-19 confirmed and the non-COVID-19 group.
A. A: COVID-19 confirmed group,
B. B: Non-COVID-19 group.
Supplemental Table 1: Clinical data analysis between the COVID-19 confirmed and the non-COVID-19 group.
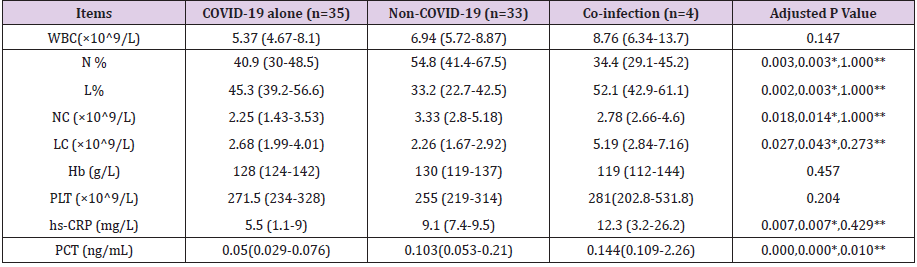
Compared to the non-COVID-19 (n=33) and co-infection (n=4) groups, the COVID-19 alone (n=35) group had significant statistical differences in PCT (0.05[0.029-0.076] vs 0.103[0.053- 0.21];P=.000 and 0.144[0.109-2.26];P=.010), percentage of neutrophil (N%) (40.9[30-48.5] vs 54.8[41.4-67.5];P=.003), percentage of lymphocyte (L%) (45.3[39.2-56.6] vs 33.2[22.7- 42.5];P=.003), neutrophil count (NC) (2.25[1.43-3.53] vs 3.33[2.8- 5.18];P=.014), lymphocyte count (LC) (2.68[1.99-4.01] vs 2.26 [1.67-2.92];P=.043), high sensitivity C-reactive protein (hs-CRP) (5.5[1.1-9] vs 9.1[7.4-9.5];P=.007). While white blood cell (WBC) count, hemoglobin (Hb) and platelet (PLT) were statistically insignificant (Table 2). We grouped coinfections and non-COVID-19 infections into one category and used binary logistic regression analysis to screen independent differential diagnostic indicators for COVID-19 alone infections. WBC, N%, L%, NC, LC, hs-CRP and PCT were entered into a backward stepwise multivariate logistic regression model, and the last step was to obtain two independent indicators of NC (P=0.082) and PCT (P=0.000) (Table 3). Goodness of fit testing (Hosmer-Lemeshow test) was used to assess deviations between observed and expected values. A P value of >.05 implies no significant difference between the observed and expected values. The P value of the goodness-of-fit testing of our model is 0.803, and therefore it is acceptable (Figure 1). Analysis of the AUC of the ROC curve was constructed to assess the differentiating performance. The AUC of overall model is 0.834 ([95%CI][0.741-0.926];P=.000). PCT as a differential diagnostic indicator of COVID-19 alone; its AUC is 0.809 ([0.710-0.909];P=.000) (Figure 1 & Table 4). Considering the principle of practicability and good accuracy, the PCT cutoff value is optimized and adjusted to 0.1ng/mL, the sensitivity, specificity, PPV, and NPV of differentiating are 65.9%, 78.6%, 82.9%, and 59.2% (Table 5).
Table 2: Comparison of laboratory data between the three groups.
Note: *COVID-19 alone Vs Non-COVID-19; **COVID-19 alone Vs Co-infection.
Discussion
Every winter and spring, a variety of virus infections are prevalent worldwide, such as parainfluenza, RSV, INF A and B, rhinovirus, and cytomegalovirus, etc [12,13]. A significant number of communities acquired pneumonia (CAP) are caused by viruses, either directly or as part of a co-infection. The clinical picture of these different pneumonias can be very similar, but viral infection is more common in the pediatric populations. Despite great advances in virus detection and pneumonia imaging [4], the identification and differential diagnosis of viral infections has been facing enormous challenges because laboratory detections and radiographic images are not always in agreement with clinical features [14,15]. RTPCR methods based on spike gene and N gene were widely used for detecting SARS-CoV-2 and are considered a gold standard for COVID-19 confirmations [16-18]. However, this method has its limitations, such as false positive or false negative results, incorrect sampling, inconsistency of sample collection and preparation, and it is time consuming. Most importantly, the inaccuracy of RT-PCR method will cause problems in timely triage, isolation of the source of infection, subsequent treatment decisions, and may even lead to errors in the prevention and control of COVID-19. In addition, CT changes in novel coronaviral pneumonia are nonspecific and difficult to differentiate from other viral infections [10,15]. Doing these CT scans in children also comes with disadvantages, such as high costs, the need for sedation, and radiation exposure.
Buonsenso et al suggested that the routine and indiscriminate CT scans for children may be clinically and ethically inappropriate [16]. Recent evidence indicates the usefulness of lung ultrasound (LUS) in detecting COVID-19 pneumonia and its non-inferiority to chest CT scan [17-19]. For this reason, some researchers are suggesting the routine use of LUS in the evaluation of children with suspected or confirmed COVID-19 [19]. As a result, it is very necessary to start with other clinical features and laboratory data, independent of nucleic acid detection and chest CT, to provide a basis for early differential diagnosis. COVID-19 as a viral infection; it is obvious that children are especially susceptible. Most of the infected children that we observed are asymptomatic or mildly febrile and/or display symptoms of ARI [3,20]. However, the changes of chest CT are very typical of viral pneumonia, which is consistent with the results of other relevant reports [15]. Also, the COVID-19 cases with co-infection all had fever and symptoms of ARI, and chest CT findings of viral pneumonia were also typical. These results suggested COVID-19 with other viral co-infections may be more common in children [13]. Once severe symptoms appear, the possibility of co-infection should be ruled out. Compared with other non-bacterial pathogen infections, COVID-19 has more family cluster infections, which indicates that the virus is more infectious and has the ability of sustained human-to-human transmission.
Therefore, disinfection of droplets and the household
environment, and hand hygiene must be the top priority for
developing preventive and control measures for children [21].
For the last two decades, most of studies have been carried out by
using WBC count, and serum hs-CRP and PCT concentration, either
alone or in combination [22,23]. PCT appears to be most effective
in selecting bacterial cases and assessing severity. However, the
precise cut-offs between the separation of bacteria from viruses
and between the separation of mild from severe cases have not
been established [24]. In adults, the normal reference value of PCT
is less than 0.1 ng/mL. Between 0.1 and 0.25 ng/mL represents
a viral infection. Between 0.25 and 0.5 ng/mL, bacterial infection
or bacterial co-infection is less likely, and antibiotic treatment is not required. Data regarding children, despite being limited, are
consistent with those collected in adults. Li Z et al found that serum
PCT level could provide a useful method of distinguishing bacterial
co-infections from an H1N1 influenza infection alone in children
during the early disease phase [25]. Chen ZM et al also suggested
using a PCT > 0.5 ng/mL to identify COVID-19 with bacterial coinfections
[11]. Our investigation also used the criterion of PCT > 0.5
ng/mL to exclude bacterial infection or co-infection. Three cases in
the non-COVID-19 group were considered bacterial infections, and
one case in the COVID-19 confirmed group had IFN B co-infection,
but the possibility of combined bacterial coinfection cannot be
ruled out.
Interestingly, the PCT of the other three cases with RSV coinfection
in the COVID-19 confirmed group was still significantly
higher than that of COVID-19 alone [26-28]. Due to limited
laboratory testing methods and personnel during the epidemic, our
hospital could only perform RT-PCR detection of IFN A, B, RSV and
cytomegalovirus except for SARS-CoV-2. Unidentified nonbacterial
infections may be other types of viral infections. It also does not
rule out that some COVID-19 cases may have these other viral coinfections.
There are several limitations in our retrospective cohort
study. First is the small sample size of the single-center research
hospital; in particular, there were only four cases in the co-infection
group. Second, the pediatric patients may be in different stages of
disease when they are admitted to the hospital. Therefore, these
results should be carefully interpreted owing to potential selection
bias and residual confounding. Larger cohort studies from other
cities in China and other countries may also be needed to provide
further data support.
Conclusion
Asymptomatic or mild symptoms, positive CT findings and family cluster infection are the main clinical features of COVID-19 in children. With good performance, PCT can provide an important basis for differentiating COVID-19 alone and other viral infections or viral co-infections.
Acknowledgement
The authors thank Cindy Acon Chen, University of Maryland School of Medicine, USA, for reviewing this manuscript. We also thank all the participants, their families, and the institutions for supporting this study. This manuscript has been released as a pre-print at [medRxiv 2020.04.07.20057315; doi: https://doi. org/10.1101 /2020.04.07.20057315], (Denggao Peng et al.).
Conflict of Interest
No conflicts of interest to disclose.
Author Contributions
• Concept and design: DP, JZ
• Data curation: PW, ZL
• Writing - original draft: ZL, PW
• Writing - review & editing: DP, JZ
• Approval of the final manuscript: DP, JZ, ZL, PW.
References
- Li Q, Guan X, Wu P, Wang X, Zhou L, et al. (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med 382(13): 1199-1207.
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506.
- Dong Y, Mo X, Hu Y, Qi X, Jiang F, et al. (2020) Epidemiological Characteristics of 2143 Pediatric Patients With 2019 Coronavirus Disease in China. Pediatrics pii: e20200702.
- Wang Y, Wang Y, Chen Y, Qin Q (2020) Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol 92(6): 568-576.
- Buonsenso D, Zampino G, Valentini P (2020) Novel Coronavirus Disease 2019 Infection in Children: The Dark Side of a Worldwide Outbreak. Front Pediatr 8: 215.
- Götzinger F, Santiago García B, Noguera Julián A, Lanaspa M, Lancella L, et al. COVID-19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc Health 4(9): 653-661.
- Parri N, Lenge M, Buonsenso D (2020) Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group. Children with Covid-19 in Pediatric Emergency Departments in Italy. N Engl J Med 383(2): 187-190.
- Whittaker E, Bamford A, Kenny J, Kaforou M, Jones C, et al. (2020) Clinical Characteristics of 58 Children with a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA 324(3): 259-269.
- The guidelines for diagnosis and treatment of 2019 novel coronavirus infected pneumonia (the sixth edition draft) issued by the National Health Commission of China.
- Xia W, Shao J, Guo Y, Peng X, Li Z, et al. (2020) Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol 55(5): 1169-1174.
- Chen ZM, Fu JF, Shu Q, Chen YH, Hua CZ, et al. (2020) Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr 16(3): 240-246.
- Shi T, Balsells E, Wastnedge E, Singleton R, Rasmussen ZA, et al. (2015) Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: Systematic review and meta-analysis. J Glob Health 5(2): 020416.
- Baillie VL, Olwagen CP, Madhi SA (2018) Review on Clinical and Molecular Epidemiology of Human Rhinovirus-Associated Lower Respiratory Tract Infections in African and Southeast Asian Children. Pediatr Infect Dis J 37(7): e185-e194.
- Kawaguchi A, Bates A, Lee BE, Drews S, Garros D (2018) Virus detection in critically ill children with acute respiratory disease: A new profile in view of new technology. Acta Paediatr 107(3): 504-510.
- Fang Y, Zhang H, Xie J, Lin M, Ying L, et al. (2020) Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology 296(2): 200432.
- Buonsenso D, Parri N, De Rose C, Valentini P (2020) Toward a clinically based classification of disease severity for paediatric COVID-19. Lancet Infect Dis 21(1).
- De Rose C, Inchingolo R, Smargiassi A, Zampino G, Valentini P, et al. (2020) How to Perform Pediatric Lung Ultrasound Examinations in the Time of COVID-19. J Ultrasound Med.
- Buonsenso D, Pata D, Chiaretti A (2020) COVID-19 outbreak: Less stethoscope, more ultrasound. Lancet Respir Med 8(5): e27.
- Musolino AM, Supino MC, Buonsenso D, Ferro V, Valentini P, et al. (2020) Lung Ultrasound in Children with COVID-19: Preliminary Findings. Ultrasound Med Biol 46(8): 2094-2098.
- Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, et al. (2020) Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin Chem 66(4): 549-555.
- Cai J, Xu J, Lin D, Yang Z, Xu L, et al. (2020) A Case Series of children with 2019 novel coronavirus infection: Clinical and epidemiological features. Clin Infect Dis 1(6): 1547-1551.
- Thompson LA, Rasmussen SA (2020) What Does the Coronavirus Disease 2019 (COVID-19) Mean for Families? JAMA Pediatr 174(6): 628.
- Kotula JJ, Moore WS, Chopra A, Cies JJ (2018) Association of Procalcitonin Value and Bacterial Coinfections in Pediatric Patients with Viral Lower Respiratory Tract Infections Admitted to the Pediatric Intensive Care Unit. J Pediatr Pharmacol Ther 23(6): 466-472.
- Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J (2004) Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin Infect Dis 39(2): 206-217.
- Principi N, Esposito S (2017) Biomarkers in Pediatric Community-Acquired Pneumonia. Int J Mol Sci 18(2): 447.
- Li Z, He L, Li S, He W, Zha C, et al. Combination of procalcitonin and C-reactive protein levels in the early diagnosis of bacterial co-infections in children with H1N1 influenza. Influenza Other Respir Viruses 13(2): 184-190.
- Lu R, Zhao X, Li J, Niu P, Yang B, et al. (2020) Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395(10224): 565-574.
- Xiao SY, Wu Y, Liu H (2020) Evolving status of the 2019 novel coronavirus infection: Proposal of conventional serologic assays for disease diagnosis and infection monitoring. J Med Virol 92(5): 464-467.

 Research Article
Research Article