Abstract
Lung cancer is the most common cause of cancer-related death worldwide and the prognosis in stage IV is poor with the overall survival not exceeding one year in patients treated with chemotherapy. Molecular disturbances in tumor cells such as EGFR gene mutations (exon 19 deletion or L858R substitution in exon 21 EGFR gene) are indication to use targeted therapies. We present a case of a 31-year-old female with metastatic adenocarcinoma of the lung with the presence of compound uncommon EGFR gene mutations, in which afatinib was used in first line treatment, with a partial response successfully obtained. The treatment was initiated fifteen months ago and is still continued despite acute skin side effects at the beginning of therapy.
Keywords: Adenocarcinoma; Afatinib; Uncommon EGFR mutation
Abbreviations: TKIs: Tyrosine Kinase Inhibitors; EGFR: Epidermal Growth Factor Receptor; NSCLC: Non-Small Cell Lung Cancer; GGOs: Ground-Glass Opacities; GGNs: Ground-Glass Nodules; BAL: Bronchoalveolar Lavage
Introduction
Lung cancer is the most common cause of cancer-related deaths in the world. The prognosis of patients with advanced or metastatic lung cancer is still poor. The median survival time in patients treated with classic chemotherapy does not exceed 12 months. The presence of specific molecular alterations is a positive predictor of the effectiveness of treatment with tyrosine kinase inhibitors (TKIs). In addition to better effectiveness in this specific population as compared to standard chemotherapy, they show an improvement in the quality of life and a different safety profile. At present, first- (erlotinib and gefitinib) [1,2] second- (afatinib and dacomitinib) [3,4] and third generation (osimertinib) [5] inhibitors can be used in the first-line systemic treatment. Activating epidermal growth factor receptor (EGFR) mutations are reported in approximately 10-15% of Caucasian patients with non-small cell lung cancer (NSCLC), mostly in female, younger patients, and in non-smokers or light smokers [6]. The most frequently found EGFR gene mutation is a deletion in exon 19, representing 45% of all mutations, followed by L858R substitution in exon 21 (40- 45%) [7,8] So-called uncommon EGFR gene mutations are found in 7-23% of patients [9,10]. Compound mutations are identified in up to 25% of uncommon mutations [11]. The prognosis depends strictly on the type of mutation. Patients with the most common rare mutations (“major” uncommon), such as G719X, L861Q and S768I, or patients with compound mutations have a better prognosis, and patients with EGFR exon 20 insertion or T790M mutation have a far worse prognosis [12].
Case Report
In July 2019, a 32-year-old non-smoking female reported to her family doctor due to a dry cough lasting for a month. In May 2019, she had a miscarriage in the 10th week of pregnancy. Medical history revealed no comorbidities, and the family history was also not burdened. A chest X-ray showed bilateral shadows of 4 to 6 mm in diameter. The spiral computed tomography of the chest, performed in August 2019, confirmed the presence of multiple disseminated ground-glass opacities (GGOs) up to 15 mm in both lungs and multiple ground-glass nodules (GGNs) with dimensions up to 9 mm. Additionally, in segment 10 of the right lung, a well-saturated, nodular lesion of 20 mm was visualized. The differential diagnosis included sarcoidosis as well as inflammatory and metastatic lesions. No enlarged lymph nodes in the chest were visualized. The bronchofiberoscopy did not reveal any abnormalities. No tumor cells were found in the cytological examination of the bronchoalveolar lavage (BAL) sample. Colonoscopy and gastroscopy as well as gynecological examination were performed, showing no pathology. In September 2019, a PET-CT examination was performed, showing no lesions clearly indicating a malignant process. However, nodular lesions in the lungs were visualized, which, due to the suspicion of bronchoalveolar carcinoma, underwent further diagnosis. During video thoracoscopy, a nodular lesion located in segment 10 of the right lung was sampled for histopathological examination, which confirmed the presence of non-mucous bronchioloalveolar carcinoma. The tissue sample was submitted for molecular testing, confirming the presence of two rare EGFR gene mutations - G719X substitution in exon 18 and S768I substitution in exon 20.
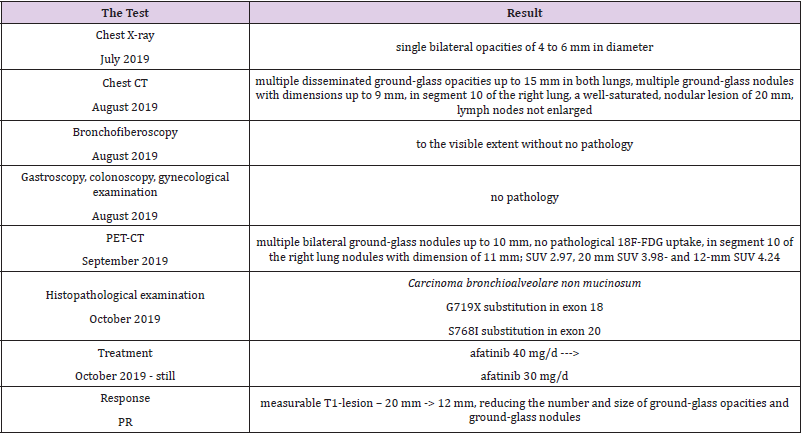
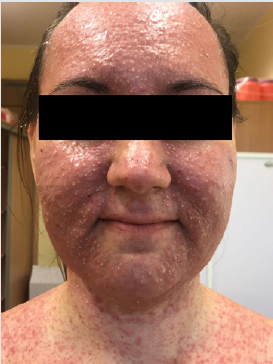
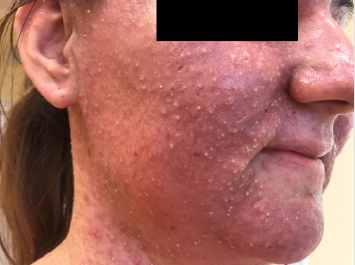
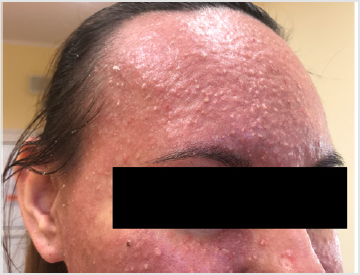
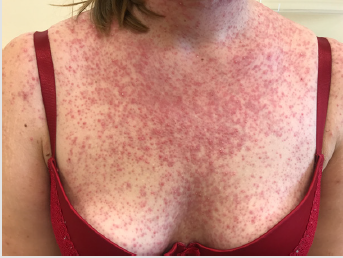
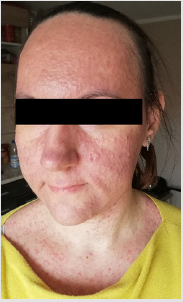
Detailed results of patient’s diagnostic tests are presented in Table 1. The patient was qualified for treatment with afatinib at daily dose of 40 mg and the therapy was initiated in October 2019. After 2 weeks of using the drug, the patient returned for a followup visit. The presence of CTCAE G3 acne-like rash accompanied by skin redness and itching was found on the skin of the face and neckline (Picture 1-4, author’s own archive). Afatinib treatment was stopped and the oral lymecycline (at a dose of 2 x 300 mg for 10 days) and topical clindamycin were administered. Antipruritic drugs were also introduced with desloratadine and dexamethasone in a dose of 4 mg for 5 days. After a week, the skin lesions began to resolve (to G2), and after 14 days the G1 acneiform rash on the face was present (Picture 5, author’s own archive). After a two-week break, treatment with afatinib was resumed at a reduced dose of 30 mg/d. In the first follow-up imaging assessment after 3 months of therapy, a partial response was found according to the RECIST (Response Evaluation Criteria in Solid Tumors) 1.1 criteria which currently retains. The patient continues treatment, there were no other side effects or recurrence of the acne-like rash. The patient is professionally active [13].
Discussion
EGFR gene mutation occurs in about 10-15% of Caucasian patients with non-small cell lung cancer6. About 90% of these mutations include exon 19 deletion (45%) and exon 21 point mutation (specifically L858R; 40-45%)7,8. Uncommon mutations account for 7-23% of all EGFR gene mutations (10% on average) and constitute a heterogeneous group of approximately 600 identified variants of molecular disorders in which the use of EGFR TKIs is associated with different efficacy9,10. The most frequently reported uncommon EGFR gene mutations include exon 20 insertions (6% of all EGFR gene mutations), mutation G719X (3%), L861Q (1%), S768I (1%) and exon 19 insertions (0.6 %) [14]. It is estimated that approximately 25% of uncommon mutations coexist with another mutation within the same tumor (so called compound mutations)11. Afatinib is an oral small molecule irreversible ErbB family receptor tyrosine kinase. Its efficacy and safety in patients with EGFR mutant (ex19del, L858R) non-small cell lung cancer has been confirmed in multicenter, randomized phase 3clinical trials LUX-Lung-3 (global study) and LUX -Lung 6 (Asian countries) [15,16]. The LUX-Lung-3 clinical trial showed an increase in median progression-free survival compared to standard first-line platinumbased chemotherapy.
In the total population of patients enrolled in the study, mPFS was 11.1 months and 6.9 months for afatinib and chemotherapy, respectively, and in the subgroup of patients with common mutations (ex19del, L858R), 13.6 months and 6.9 months, respectively (HR 0.47, P <0.0001). In patients with EGFR exon 19 deletion treated with afatinib in the LUX-Lung 3 trial, overall survival increased by more than 12 months compared to standard chemotherapy (mOS 33.3 vs 21.1 months) with a reduction in the risk of death by 46% (HR 0.54)15. In 2015, an analysis of the efficacy of afatinib was performed in patients with uncommon mutations participated in the LUX-Lung 2, LUX-Lung 3 and LUX-Lung 6 clinical trials (N=100, 75% of them were treated with afatinib). The uncommon mutations were divided into 3 groups: 1 - point mutations or exon 18-21 duplications (n=38), 2 - de novo T790M mutation in exon 20 (as the only mutation or coexisting with another mutation) (n=14) and 3 - exon 20 insertions (n=23). The prognosis of patients in the first group was definitely the best (ORR 71.1%, mPFS 10.7 months, mOS 19.4 months), while the results of afatinib treatment in groups 2 and 3 were much worse: de novo T790M - ORR 14.3%, mPFS 2.9 months, mOS 14.9 months and exon 20 insertion - ORR 8.7%, mPFS 2.7 months and mOS 9.2 months. In addition, in group 1 the results of afatinib treatment were presented according to the presence of 3 most frequent mutations distinguished: G719X, L861G and S768I (alone or co-occurring with other mutations). In patients with G719X mutation, ORR was 77.8%, median PFS was 13.8 months, and median OS was 26.9 months. For the L861G and S768I mutations, the ORR was 56.3% and 100%, respectively, mPFS 8.2 months and 14.7 months, and mOS 17.1 months, and not estimable [17].
In 2019, the pooled results of the analysis of data from 693 patients harboring EGFR gene uncommon mutations treated with afatinib within randomized clinical trials, compassionate-use and expanded-access programs, phase IIIb trials, noninterventional trials, caser series or case studies were published. The patients were divided into subgroups: 1 - patients with T790M mutation, 2 - patients with exon 20 insertion, 3 - patients with “major” uncommon mutations (G719X, L861Q, S768I), 4 - patients with compound mutations and 5 - patients with other uncommon mutations. The best results of afatinib treatment were reported in patients with major uncommon mutations (median TTF 10.8 months, ORR 60%, mDOR 17.1 months) and compound mutations (mTTF 4.5 months, ORR 65.2%, mDOR 16.6 month). In the presented patient two major uncommon mutations were identified: exon 18 G719X substitution and exon 20 S768I substitution [18]. Treatment started 15 months ago, the patient achieved a partial response according to RECIST 1.1 criteria, which currently retains. One of the most common side effects of afatinib is acneiform rash: it occurred in 89.1% of patients treated with afatinib in the LUX-Lung 3 clinical trial and in 80.8% of Asian patients treated in the LUX-Lung 6 trial. The rash grade ≥3 according to the CTCAE criteria was present in 16.2% and 14.2% of patients in the LUX-Lung 3 and LUX-Lung 6 trials, respectively. Acneiform rash was a reason for discontinuing of afatinib therapy in 2.1% of patients in the LUX-Lung 6 study and no patients in the LUX-Lung 3 study. In the presented patient the grade 3 rash resolved after the temporary discontinuation of afatinib therapy, the use of topical and oral antibiotics, and thereafter was successfully resumed at a reduced dose (Figures 1-5).
Figure 1: Acne-like rash (G3) after 2 weeks of afatinib treatment in patient with EGFR mutant NSCLC.
Figure 2: Acne-like rash (G3) after 2 weeks of afatinib treatment in patient with EGFR mutant NSCLC.
Figure 3: Acne-like rash (G3) after 2 weeks of afatinib treatment in patient with EGFR mutant NSCLC.
Figure 4: Acne-like rash (G3) after 2 weeks of afatinib treatment in patient with EGFR mutant NSCLC.
Figure 5: Acne-like rash (G2) one week after afatinib discontinuation and introducing antibiotic therapy.
Summary
Afatinib is an effective and safe therapeutic option in lung cancer patients with certain uncommon EGFR gene mutations. The presented case is an example of a long-term response to afatinib. In addition, it emphasizes the need for close monitoring of patients in terms of possible side effects, including dermatological complications. Treatment with EGFR TKIs maintains a good quality of life, and possible side effects are usually transient and manageable.
References
- Zhon C, Wu YL, Chen G, Feng J, Qing Liu X, et al. (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicenter, open-label, randomized, phase 3 study. The Lancet Oncology 12(8): 735-742.
- Sequist L, Martins R, Spigel D, Grunberg SM, Spira A, et al. (2008) First line gefitinib in patients with advanced non-small-cell lung cancer harbouring somatic EGFR mutations. Journal of Clinical Oncology 26(15): 2442-2449.
- Yang CH, Wu YL, Schuler M, Sebastian M, Popat S, et al. (2015) Afatinib versus cisplatin-based chemotherapy for EGFR mutation positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomized, phase 3 trial. The Lancet Oncology 16(2): 141-151.
- Wu YL, Cheng Y, Zhou X, Lee K, Nakagwa K, et al. (2017) Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomized, open-label, phase 3 trial. The Lancet Oncology 18(11): 1454-1466.
- Ramalingam S, Vansteenkiste J, Planchard D, Chul Cho B, Gary JE, et al. (2020) Overall survival with Osimertinib in untreated, EGFR-mutated advanced NSCLC. NEJM 382: 41-50.
- Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR, et al. (2016) The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget 7(48): 7898578993.
- Kobayashi S, Canepa HM, Bailey A, Nakayama S, Yamaguchi Y, et al. (2013) Compound EGFR mutations and response to EGFR tyrosine kinase inhibitors. Journal of Thoracic Oncology 8: 45-51.
- Kim EY, Cho EN, Park H, Hong JY, Lim S, et al. (2016) Compound EGFR mutation is frequently detected with co-mutations of actionable genes and associated with poor clinical outcome in lung adenocarcinoma. Cancer Biol Ther 17(3): 237-245.
- Keam B, Kim DW, Park JH, Lee JO, Kim TM, et al. (2014) Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non-small cell lung cancer. Int J Clin Onco 19: 594-600.
- Krawczyk P, Kowalski DM, Ramlau R, Warzocha Ek, Winiarczyk K, et al. (2017) Comparison of the effectiveness of erlotinib, gefitinib, and afatinib for treatment of non-small cell lung cancer in patients with common and rare EGFR gene mutations. Oncology Letters 13: 4433-4444.
- Kohsaka S, Nagano M, Ueno T, Suehara Y, Hayashi T, et al. (2017) A method of high-throuhgput functional evaluation of EGFR gene variants of unknown significance in cancer. Sci Transl Med 21: 571-579.
- Ahn MJ, Cho JH, Sun JM, Kee SH, Ahn JS, et al. (2018) An open-label, multi-center, phase II single arm trial of osimertinib in non-small cell lung cancer patients with uncommon EGFR mutation (KCSG-LU15-09). J Clin Oncol 36: 9050.
- Passaro A, Prelaj A, Bonanno L, Tiseo M, Tuzi A, et al. (2019) Activity of EGFR TKIs in Caucasian patients with NSCLC harbouring potentially sensitive uncommon EGFR mutations. Clin Lung Cancer 20: e186-e194.
- Kobayashi Y, Mitsudomi T (2016) Not all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategy. Cancer Sci 107: 1179-1186.
- Sequist L, Yang J, Yamamoto N, O Byrne K, Tony Mok VH, et al. (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. Journal of Clinical Oncology 31(27): 3327-3334.
- Wu YL, Zhou C, Hu CP, Feng J, Lu S, et al. (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncology 15(2): 213-222.
- Yang J, Sequist L, Geater S, Tsai CM, Kam Mok TS, et al. (2015) Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncology 16: 830-838.
- Yang J, Schuler M, Popat S, Miura S, Heeke S, et al. (2019) Afatinib for the treatment of NSCLC harboring uncommon EGFR mutations: a database of 693 cases. Journal of Thoracic Oncology 15(5): 803-815.

 Case Report
Case Report