Abstract
Abbreviations: SLL: Staple Line Leack; LSG: Laparoscopic Sleeve Gastrectomy; BMI: Body Mass Index
Introduction
The purpose of this analysis is to identify a therapeutic algorithm in case of Staple Line Leack (SLL) after Laparoscopic Sleeve Gastrectomy (LSG) based on the evidence present in the literature and our clinical experience. Sleeve gastrectomy (LSG) is a surgery procedure practiced to treat morbigena obesity. The anthropo-pylon region and the vassal innervation remain intact. The intervention is carried out mainly in laparoscopy [1,2]. Several complications may occur after sleeve gastrectomy [3,4] (Table 1). SLL is the most worrying and potentially deadly complications of this procedure. A leak is defined as leaking gastrointestinal content through a continuous solution of the sleeve suture line, which can freely carry itself into the peritoneal cavity or gather next to the suture [5,6]. SLL can be classified based on the time of appearance of the SLL, site, clinical presentation [1,4,7].
Based on the timing Regimbeau [8] classify SLL as:
a) Early (appearance within the first week)
b) Late (appearing after the first week).
Based on the site of dehiscence:
a) Superior
b) Average
c) Distal.
Based on the clinical presentations:
a) Systemic signs such as inflammation and sepsis (tachycardia, hyperthermia)
b) Peritonitis
c) Pulmonary symptoms
d) Intra-abdominal abscess.
Data
We report a series of patients who presented SLL after LSG among 296 patients undergoing LSG between January, 2008 and January, 2017 at our operations unit. Patients who had undergone other bariatric surgery before LSG were excluded. The following data were collected: epidemiological characteristics, comorbidity, timing of the appearance of SLL in relation to LSG, the symptomatological framework of onset, the instrumental examinations performed.
Among the patients analyzed, 5 (1.65%) were lost at the follow-up and 9 had a SLL (3.04%):
• 4 early (1.35%)
• 5 late (1.69%).
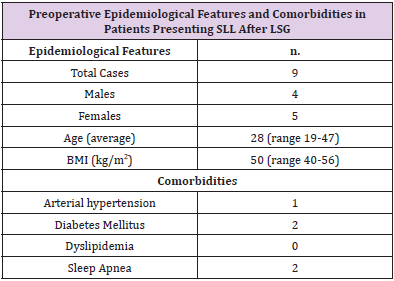
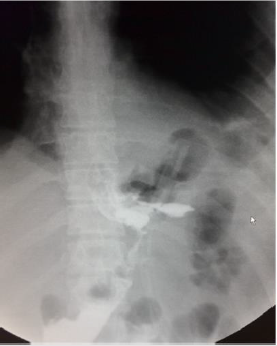
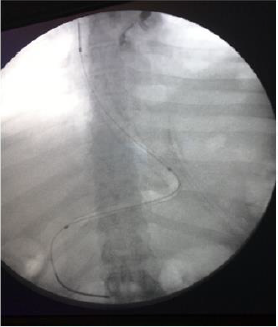
The average age of patients who submitted SLL was 28 years (range 19 - 47), the average BMI 50 kg/m2 (range 40 - 56) (Table 1). All leaks showed up at the next portion of the stomach, below the gastroesophageal junction. No deaths have occurred. Diagnosis of SLL was carried out in an average period of 37 days (range, 1-121). Symptoms of onset were: hyperpiressia with chills (n=8), abdominal pain (n=2) nausea and vomiting (n=1). Diagnosis was made with abdomen CT with c.m. (n=6), Esophagus-stomach XR with water-soluble c.m. (n=3) (Figure 1). In one case the diagnosis was made by means of routine radiological control, without having specific symptoms and/or signs. A multidisciplinary approach was used for all patients, including fasting, total parenteral nutrition and antibiotic therapy.
Table 2: Synopsis of our experience in managing a series of staple line leaks post sleeve gastrectomy.
Etiopathogenesis
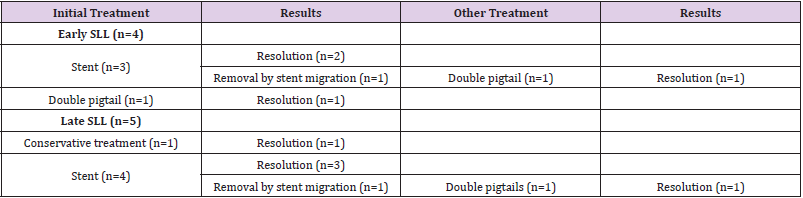
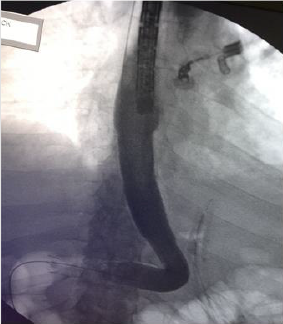
In all cases of late SLL, we placed a guided CT drainage to drain the perigastric purulent collection. In a case it was necessary a re-surgery conducted in laparoscopy for the persistence of hyperpiressia and the appearance of signs of sepsis despite the drainage placed under CT guidance. During the intervention, washing of the perigatric area and drainage of the collection was carried out. Conservative treatment (fasting, parenteral nutrition, antibiotic therapy, nasogastric sondino) was used in one patient. Endoscopic positioning of megastent was used in seven patients underwent with (n=1) or without (n=6) pylon expansion. In one case it was necessary to place a second stent after 4 weeks to persist, although reduced, of the leak and after the removal of the second stent the leak had healed. In another case it was necessary to replace the stent by the appearance of disphagia after two weeks, the removal stent had a stenosis in its distal part (Figure 2). Five patients who had a stent placed obtained a leak healing in an average time of 42.8 days (range, 28-84).
In two cases, instead, it was necessary to remove the stent for dislocation of the same and reappeared hyperpiressia and a new perigatric collection. In these caseses, a transgastric drainage with double pigtails was realized at the same time. In one patient, transgastric drainage was used as the initial and only treatment. In these three cases two transgastrial endoprosthetics were placed at the leakage point, two double pygmies with a diameter of 8.5 fz and length 3 cm. The patients were fed for two weeks through an enteric nose tube then they gradually resumed oral feeding. After 90 days the CT scan showed that the prostheses were no longer on site and there were no more signs of SLL. One of them at the CT check at 3 months still had one of the double pigtails in place so it was necessary to remove it endoscopically (Table 2).
Discussion
SLL is one of the most worrying complications of LSG. The incidence varies from 2.4% to 7% depending on the case [8- 10]. Most leaks appear at the upper portion stalpe line, near the gastroesophageal junction or near the corner of His [11]. Burgos, et al. [12] reported that 85.7% of leaks is at the level of the subcardial region and that only 14.3% are distal. According to a metaanalysis of Saber [10] which considered 29 jobs for a total of 4888 patients (BMI between 34 and 65.4 kg/m2) the average incidence of SLL is 2.4% (range, 0-7%). Many studies show that the incidence in the super-obese (BMI > 50 Kg/m2) is 2.9 %, while the average incidence in non-super obese patients (BMI < 50 Kg/m2) is 2.2% [13]. Probably the leaks of the upper portion of the suture line have a multifactorial etiology. Some AAs have formulated the mechanistic theory that a suture too close to the esophagus-gastric junction and/or abdominal esophagus results in increased tension on the suture line and is this tension according to this theory causing the increased incidence of SLL in this area [14]. A second theory is that so called vascular, according to it the section of the gastric bottom would determine a relative ischemia at the expense of a critical area of the next portion of the suture line that would increase the risk of SLL [15]. Basso, et al. [16] describe well the spraying of this area indicating its critical issues.
The cardias is sprayed to the right and front by branches of the left gastric artery and the lower left phrenic artery, the left posterior portion is vascularized mainly by fundic branches of the splenic artery and, if present, by the posterior gastric artery. Spraying of the esophagus is segmental. Complete dissection of the gastric background requires dissection of short vessels, posterior gastric artery and the brake branch when present. This creates a critical area of vascularization laterally, at the level of the esophagusgastical junction and the Angle of His. To avoid SLL, a resection line has been proposed by many AAs that avoids the critical area, it is obtained by leaving a gastric residue of 1-2 cm precisely at the level of the gastroesophageal junction [17]. A further theory sees as the cause of stenosis at the level of angular incisura in fact Yehoshua, et al. [18] has shown that high intraluminal pressure and low compliance of the gastric tube (situation that is determined when there is a stenosis to aungulus) can be the cause of leaks. There are also, described in the literature, numerous factors related to the patient that relate to a higher incidence of leaks. They are advanced age, BMI> 60 kg/m2, malnutrition and history of gastric bandage. The typical ischemic SLL appears between the fifth and sixth days after LSG, when the healing process of the gastric wall is between the inflammatory and fibrotic phase. When the cause is mechanicaltissue fistulas usually occur before this period, in the first and second days [19].
Diagnosis
Early diagnosis is critical for therapy. Tachycardia of unknown origin, fever, abdominal pain or persistent sob should alarm the surgeon. Anyway, the clinical presentation of SLL can vary from the completely asymptomatic patient to the one with peritonitis, septic shock, multiorgan failure and death [20]. Burgos, et al. [12] reports a series of 7 leaks in 214 patients (3.3%) of which 5 had abdominal pain, fever, tachycardia, tachypnea, and increased laboratory infection values. It has been observed that tachycardia is an initial sign of early leak. Patients with early SLL often show signs of sepsis caused by the spreading of the gastrointestinal content in the peritoneal cavity in a widespread way and surgical washing with drainage may be necessary then a re-intervention. In patients with late SLL, gastroenteric material usually accumulates perigastrically and does not spread to the rest of the abdominal cavity [21].
Treatment
Treatment options include:
a) Surgery (laparoscopic or laparotomic which consists of abdominal washing and drainage of the collection) [22].
b) Endoscopy (endoprosthesis: stent, double pigtails, etc.)
c) Radiology (percutaneous drainage, etc.).
Leak management depends on the patient’s clinical condition. The surgeon handling the complication must have a clear strategic algorithm based on the patient’s condition, leak duration and available resources [23,24]. .
Discussion
The number of LSG has grown enormously in recent years. Today LSG is the most practiced bariatric surgery in the world [2,25]. Despite this, managing some of the LSG complications still appears to be a challenging challenge for the surgeon. SLL is one of these fearsome complications [7]. It is known from the literature that the SLL of the proximal portion of the suture line has a much greater incidence and our data agree. The incidence of post-LSG SLL varies from 2.4% to 7% depending on the case [8-10]. In our analysis the incidence was 3.1%. Some surgeons believe that the suggitto can, by reinforcing the suture, reduce the incidence of SLL. From the analysis of our data no patients had been overtyped and therefore we cannot make a comparison. Many studies exist on the use of bovine pericaryum membranes to reinforce the suture line, some authors believe that these significantly reduce the incidence of SLL in fact there is no agreement on this [26]. For the patients included in our study, bovine pericartion reinforcement was used, we believe that these devices can reduce the incidence of bleeding but have no influence on the sealing of the suture [27]. The treatment of SLL is multidisciplinary and requires the commitment of medical, radiological, endoscopic and surgical procedures as well as the use of innovative and/or biotechnological technologies [28].
Surgery in case of SLL, generally conducted with laparoscopic method, has two fundamental indications:
1) Diagnosis of early SLL
2) Appearance of sepsis and MOF
The purposes are:
1) Peritoneal cavity washing
2) Place effective drainage
3) Pack a digiunostomy.
In our case study it was necessary to carry out only one resurgery for a late SLL in which signs and symptoms of sepsis appeared, a washing of the abdominal cavity was carried out and the packaged an effective external drainage, the intervention was conducted in laparoscopy [29]. Radiological percutaneous drainage is generally indicated in late SLL cases to drain the perigatric collection and take purulent material for coltural examination. It is a temporary measure pending endoscopic treatment. In the cases that we considered, in 4 patients with late SLL was placed the CT drainage guided before the placement of the megastent it allowed the evacuation of the perigastrica collection and the identification of targeted and effective antibiotic therapy. Endoscopic treatment as a placement of endoluminal prostheses (Megastent) is a temporary procedure to allow the fluid contents of the tube to bypass the leak site area while waiting for healing to take place for second intention. Eisendrath, et al. they show a therapeutic success rate of 75% proposing it as a treatment of choice for both low and high range SLL, emphasizing the importance of a rapid resumption of nutrition for os (liquid) and a quick home return of the patient.
The negative aspects of such a procedure lie in retrosternal pain, vomiting, nausea although these symptoms are more frequent in the first days. There is a risk of migration and stenosis of the prosthesis requiring endoscopic and/or laparoscopic emergency intervention. In our case studies the stent was used as an initial procedure in 5 patients and was enough to achieve complete resolution. Only in two cases did he migrate to the esophagus and was urgently removed by an endoscopic procedure. Endoscopic treatment with transgastric drainage positioning (double pigtail) as in the treatment of pseudocysts in case of chronic pancreatitis is indicated in case of early SLL when the stent is contraindicated and in case of late SLL. The purpose of the transgaxic prosthesis is to drain the perigatric collection towards the gastric light and at the same time promote healing for second intention. In our experience the double pigtail was successfully used as the first intervention in a patient who presented an early SLL diagnosed in the first day and in two patients after migration of the stent as a second intervention.
Currently, megastent treatment is the most practiced endoscopic approach. However, its use is discussed due to low patient tolerance and migration risk ranging from 33% to 88%. In recent times some authors (Peguignot, et al.) [30] consider treatment with first choice double pigtail, they believe that this procedure is more effective, better tolerated and results in faster healing when compared to the stent. On the contrary, it is believed that, for the purposes of healing, rather than bypassing the leak site it is more important to achieve an effective drainage of all perigatric collections. Therefore, many AA have abandoned the stent and propose the use of transgastric dredging with double pigtail as a first choice treatment of SLL.
Conclusion
SLL is LSG’s most frequent and fearsome complication and its exclusively surgical treatment has proved to be a challenging challenge for the bariatric surgeon [10,31]. We believe, and in this we are supported by numerous works present in the literature, that the treatment of SLL must avail itself of the skills of other specialties such as radiology, endoscopy, engineering, etc. [32]. According to scientific literature and our experience we [32,33] have developed our own decision-making algorithm based on our data and the evidence in the literature. We distinguish two therapeutic pathways based on two clinical frameworks present: stable patient, nonstable patient.
Stable Patient
in case of localized peritonitis, but not the signs and symptoms of sepsis there is no indication of re-intervention [34]. The treatment will consist in the placement of an endoprosthesis: a megastent with the realization of a guided CT external drainage or one or more double pigtails to achieve an in addition transgastric drainage there will be an enteral nose tube for nutrition. Both procedures aim to complete the drainage of the harvest, stimulate healing for second intention and allow a rapid resumption of oral nutrition.
Unstable Patient
there are signs and symptoms of sepsis, there is widespread peritonitis and there may be an interest in the median. In these patients is indicated the surgery of drainage and washing of the abdominal cavity, a fasting can be packaged, if necessary, a pulmonary lobectomy can be carried out. Once stabilized to the patient will be placed a transgastric drainage (one or more double pigtails) which has the function of promoting healing and helping to further drain the collection the treatment also includes an entral nose tube for one feed the patient. In conclusion, we can say that that of SLL is a minimally invasive and conservative treatment possible thanks to a modern multidisciplinary approach.
Notes
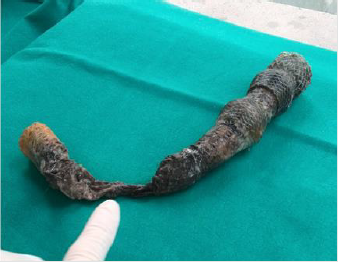
Note 1. Megastent
It is a prosthesis placed in an endoscopic procedure that is performed under general anesthesia (Figures 3 & 4), it aims to create a physical barrier to the leakage of gastric material through the leackage point through the placement of a tubular prosthesis [35].
The prosthesis used is a generally a egastent with the following characteristics:
1) Soft and flexible body that adapts to the anatomy of the tabulated neostomac.
2) Sufficient diameter and length to ensure that the extrudalal is located at the middle third of the esophagus and the distal extremity to the ladenum or trans pylon to the first duodenal portion.
3) The stent is completely covered with silicone so it can be removed with extreme simplicity and has a second metal mesh that soon anchors it even more and reduces the risk of migration.
The device is left on site for at least four weeks, but no later than six for the risk of tissue hyperplasia that would hesitate in greater difficulty during removal. Prosthesis often induces nausea, vomiting and restrosternal pain especially in the early hours and early days so much that it is little tolerated by patients [36]. There is a risk of migration and relocation that varies from 18% to 33% depending on the case [18,37].
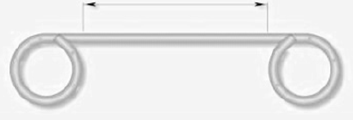
Note 2. Transgastric Drainage
It is a prosthesis placed in endoscopic procedure conducted under general anesthesia that consists in the placement of one or two small transgastric drainages in the shape of double pigtails (Figure 5) through the continuous solution of the suture line to obtain a drainage to the gastric cavity (at less pressure) of the spilled material and thus stimulate healing for second intention. It can be associated with the simultaneous placement of an enteral nose snooze for enteral nutrition. The patient will be able to resume the diet for about 15 days after the procedure. Usually after a few months the small prosthesis relocates and is expelled with feces, its removal therefore only rarely requires a new endoscopy [30].
References
- Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, et al. (2015) Bariatric Surgery Worldwide 2013.Obessurg 25(10): 1822-1832.
- Brethauer SA, Hammel JP, Schauer PR (2009) Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis 5(4): 469-475.
- Sroka G, Milevski D, Shteinberg D, Mady H, Matter I (2016) Minimizing Hemorrhagic Complications in Laparoscopic Sleeve Gastrectomy--a Randomized Controlled Trial. Obes Surg 26(2): 379-380.
- Frezza EE, Reddy S, Gee LL, Wachtel MS (2009) Complications after sleeve gastrectomy for morbid obesity. Obes Surg 19(6): 684-687.
- Peel AL, Taylor EW (1991) Proposed definitions for the audit of postoperative infection: a discussion paper. Surgical Infection Study Group. Ann R Coll Surg Engl 73(6): 385-388.
- Bruce J, Krukowski ZH, Al Khairy G, Russell EM, Park KG (2001) Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 88(9): 1157-1168.
- Douketis JD, Macie C, Thabane L, Williamson DF (2005) Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond) 29(10): 1153-1167.
- Lalor PF, Tucker ON, Szomstein S, Rosenthal RJ (2008) Complications after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis 4(1): 33-38.
- Nasser Sakran, David Goitein, Asnat Raziel, Andrei Keidar, Nahum Beglaibter, et al. (2013) Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc 27(1): 240-245.
- Aurora AR, Khaitan L, Saber AA (2012) Sleeve gastrectomy and the risk of leak: a systematic analysis of 4,888 patients. Surg Endosc 26(6): 1509-1515.
- Nicholas Dugan, Abdelrahman Nimeri (2020) Surgical Management of a Chronic Sleeve Gastrocolic Fistula with Near Total Gastrectomy and Roux-en-Y Reconstruction. Obes Surg 30(9): 3640-3641.
- Burgos AM, Braghetto I, Csendes A, Maluenda F, Korn O, et al. (2009) Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg 19(12): 1672-1677.
- Pornthep Prathanvanich, Bipan Chand (2015) Laparoscopic sleeve gastrectomy: Management of complications. Minimally Invasive Bariatric Surgery, pp 151-171.
- Ramos AC, de Souza Bastos EL, Ramos MG, Bertin NTS, Galvão TD, et al. (2015) Technical Aspects of Laparoscopic Sleeve Gastrectomy. Arq Bras Cir Dig 28(Suppl 1): 65-68.
- Chivot C, Robert B, Lafaye N, Fuks D, Dhahri A, et al. (2015) Laparoscopic sleeve gastrectomy: imaging of normal anatomic features and postoperative gastrointestinal complications. Diagn Interv Imaging 94(9): 823-834.
- Basso N, Casella G, Rizzello M, Abbatini F, Soricelli E, et al. (2011) Laparoscopic sleeve gastrectomy as first stage or definitive intent in 300 consecutive cases. Surg endosc 25(2): 444-449.
- Baker RS, Foote J, Kemmeter P, Brady R, Vroegop T, et al. (2004) The science of stapling and leaks. Obes Surg 14(10): 1290-1298.
- Yehoshua RT, Eidelman LA, Stein M, Fichman S, Mazor A, et al. (2008) Laparoscopic sleeve gastrectomy--volume and pressure assessment. Obes Surg 18(9): 1083-1088.
- Rebibo L, Darmon I, Peltier J, Dhahri A, Regimbeau JM (2017) Gastropancreatic ligament: Description, incidence, and involvement during laparoscopic sleeve gastrectomy. Clin Anat 30(3): 336-341.
- Jurowich C, Thalheimer A, Seyfried F, Fein M, Bender G, et al. (2011) Gastric leakage after sleeve gastrectomy-clinical presentation and therapeutic options. Langenbecks Arch Surg 396(7): 981-987.
- Tan JT, Kariyawasam S, Wijeratne T, Chandraratna HS (2010) Diagnosis and management of gastric leaks after laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg 20(4): 403-409.
- Ruiz Tovar J, Oller I, Llavero C, Arroyo A, Muñoz JL, et al. (2013) Pre-operative and early post-operative factors associated with surgical site infection after laparoscopic sleeve gastrectomy. Surg Infect (Larchmt) 14(4): 369-373.
- De Aretxabala X, Leon J, Wiedmaier G, Turu I, Ovalle C, et al. (2011) Gastric leak after sleeve gastrectomy: analysis of its management. Obes Surg 21(8): 1232-1237.
- Csendes A, Braghetto I, León P, Burgos AM (2010) Management of leaks after laparoscopic sleeve gastrectomy in patients with obesity. J Gastrointest Surg 14(9): 1343-1348.
- Carlin AM, Zeni TM, English WJ, Hawasli AA, Genaw JA, et al. (2013) The comparative effectiveness of sleeve gastrectomy, gastric bypass, and adjustable gastric banding procedures for the treatment of morbid obesity. Ann Surg 257(5): 791-797.
- Gill RS, Switzer N, Driedger M, Shi X, Vizhul A, et al. (2012) Laparoscopic sleeve gastrectomy with staple line buttress reinforcement in 116 consecutive morbidly obese patients. Obes Surg 22(4): 560-564.
- Simon TE, Scott JA, Brockmeyer JR, Rice RC, Frizzi JD, et al. (2011) Comparison of staple-line leakage and hemorrhage in patients undergoing laparoscopic sleeve gastrectomy with or without Seamguard. Am Surg 77(12): 1665-1668.
- Casella G, Soricelli E, Rizzello M, Trentino P, Fiocca F, et al. (2009) Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg 19(7): 821-826.
- Gehrer S, Kern B, Peters T, Christoffel-Courtin C, Peterli R (2010) Fewer nutrient deficiencies after laparoscopic sleeve gastrectomy (LSG) than after laparoscopic Roux-Y-gastric bypass (LRYGB)-a prospective study. Obes Surg 20(4): 447-453.
- Pequignot A, Fuks D, Verhaeghe P, Dhahri A, Brehant O, et al. (2012) Is there a place for pigtail drains in the management of gastric leaks after laparoscopic sleeve gastrectomy? Obes Surg 22(5): 712-720.
- Rebibo L, Delcenserie R, Brazier F, Yzet T, Regimbeau JM (2016) Treatment of gastric leaks after sleeve gastrectomy. Endoscopy 48(6): 590.
- Mauro Montuori, Domenico Benavoli, Stefano D’ugo, Luca Di Benedetto, Emanuela Bianciardi, et al. (2017) Integrated Approaches for the Management of Staple Line Leaks following Sleeve Gastrectomy. Journal of Obesity 2017: 4703236.
- Bellanger DE, Greenway FL (2011) Laparoscopic sleeve gastrectomy, 529 cases without a leak: short-term results and technical considerations. Obes Surg 21(2): 146-150.
- Casella G, Soricelli E, Rizzello M, Trentino P, Fiocca F, et al. (2009) Nonsurgical treatment of staple line leaks after laparoscopic sleeve gastrectomy. Obes Surg 19(7): 821-826.
- Nguyen NT, Nguyen XM, Dholakia C (2010) The use of endoscopic stent in management of leaks after sleeve gastrectomy. Obes Surg 20(9): 1289-1292.
- Serra C, Baltasar A, Andreo L, Pérez N, Bou R, et al. (2007) Treatment of gastric leaks with coated self-expanding stents after sleeve gastrectomy. Obes Surg 17(7): 866-872.
- Slim R, Smayra T, Chakhtoura G, Noun R (2013) Endoscopic stenting of gastric staple line leak following sleeve gastrectomy. Obes Surg 23(11):1942-1945.

 Case Report
Case Report