Abstract
This study was performed to investigate the effects of dietary turmeric powder, ginger powder, fenugreek seeds powder, dried lemon powder on antioxidant enzymes and total immunoglobulins and their fractions of growing rabbits. A total of 30, New Zealand White rabbits (NZW) at 5 weeks of age were randomly assigned to five treatments with three replicates. The dietary treatments consisted of 5 groups as follows; the basal diet as control, phytogenic additives groups were supplemented with 0.5% turmeric powder, 0.5% ginger, 1.0% fenugreek seeds and 1.0% dried lemon added to the basal diet. The data revealed that, there were there were significant differences (p≤0.01) among all dietary treatments on the values of catalase (CAT), glutathione peroxidase (GSH-Px) and Malondialdehyde (MDA). However, there were no significant differences (p>0.05) among all dietary treatments on glutathione (GSH) and Hepatic Superoxide dismutase (SOD). Rabbits fed dietary lemon recorded the highest (p≤0.01) value of Tig, IgG and IgM followed by fed on fenugreek seeds. Rabbits fed control diet recorded the lowest values of Tig and IgG compared with supplemented diets.
Keywords: Rabbits; Immunoglobulins; Antioxidant Enzymes
Abbreviations: NZW: New Zealand White Rabbits; GSH-Px: Glutathione Peroxidase; MDA: Malondialdehyde; SOD: Superoxide Dismutase; GSH: Glutathione, CAT: Catalase; Ca: Calcium; P: Phosphorus; Mg: Magnesium; K: Potassium; S: Sulfur; Na: Sodium; Fe: Iron; Mn: Manganese; Ni: Nickel; B: Boron; Si: Silicon; Cu: Copper; Zn: Zinc; Mo: Molybdenum; Se: Selenium; Co: Cobalt ; Cr: Chromium ; Ge: Germanium; As: Arsenic; Tig: Immunoglobulin; IgG: Immunoglobulin G; IgM: Immunoglobulin; IgA: Immunoglobulin A
Introduction
A part of rabbit farms profitability depends on the efficacy of weanling rabbits to grow efficiently and to rescue from morbidity and mortality during the fattening period. For this reason, antibiotic growth promoters are commonly included in the diets of growing rabbits [1]. In last decades, there are increasing concerns about using natural feed additives as antibiotic alternatives for decreasing development of antimicrobial resistance bacteria and for producing safer animal products with minimal antibiotic residues. Of natural feed additives, those from phytogenic source including different parts of the plants or their extracts are being increasingly included in animal nutrition due to their impressive range of phytochemicals. This enables them to be potentially antimicrobial candidates with multiple mechanisms of action [2]. Additionally, phytogenic compounds are not only used to control pathogenesis, but also they have been reported to improve appetite, intestinal microflora, immune functions, oxidative status, growth and carcass traits when included in animal diets [3]. Considering the affordability of these plant materials, they could be widely used as growth promoters in the livestock sector worldwide [4]. Most of the active phytochemicals identified in plant materials are of alkaloids, terpenes, flavonoides and glucosinolates [4,5]. However, each plant has a unique combination of these phytochemicals, and therefore their biological effects are expected to be different.
Among potential phytogenic feed additives, propolis is a product of plant resinous substances collected by honeybees. It has substantial levels of phenolic compounds including flavonoids, vitamins, minerals, and enzymes [6]. It has been found to have strong antioxidant, anit-inflammatory and immunomodulation activities [7]. Also, Moringa oleifera Lam is a tropical/subtropical plant with a highly nutritive value [8]. Each part of this plant has been reported to contain considerable biologically active phytochemicals particularly glucosinolates [9], and thus promising therapeutic properties [10,11]. As recently reviewed by [3] the potential biological effects of different combinations of phytochemicals on growth performance, antioxidant and antibacterial activities and blood metabolites in rabbits are not fully scrutinized. The aim of this study was to evaluate the consequence of adding some natural feed additives i.e. turmeric, ginger, fenugreek and dried lemon to rabbit diets at the levels of 0.5, 0.5, 1.0, and 1.0% respectively, on antioxidant enzymes and total immunoglobulins and their fractions of growing rabbits.
Materials and Methods
Experimental Animals
A total number of 30 males,5 weeks old growing New Zealand white rabbits were used to study the effect of some natural feed additives on antioxidant enzymes and total immunoglobulins and their fractions of growing rabbits.. Rabbits distributed into (5 treatments x 3 replicates x 2 rabbit = 30 rabbits). All rabbits were housed in open house. The rabbits were allocated in a cage with slatted floor of iron. The dimensions of the cage were (45 × 45 × 38cm) for length, width and high, respectively. Feed and water given to the rabbits ad-libitum during the experimental periods.
Experimental Diets
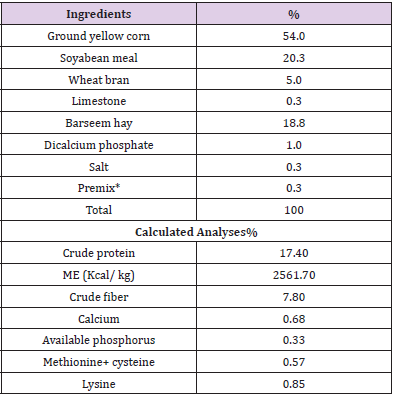
Growing rabbits were distributed to five dietary treatment groups. The first group fed control diet formulated to contain adequate levels of nutrients for growing New Zealand White rabbits as recommended by the National Research Council [12]. The formulation and chemical composition of control diet is shown in (Table 1). Chemical analysis of ingredients and diets was determined according to [13]. Four additional dietary treatment groups were formulated to contain control diet incorporated with feed supplementation according to the source of addition such as 0.5% curcuma, 0.5% ginger, 1.0% fenugreek and 1.0% dried lemon respectively.
Table 1: The composition and chemical analysis of the control diet for growing New Zealand White rabbits.
Assay of Antioxidant Biomarkers in the Tissues and Serum
Samples were removed from −80 °C storage, diluted (v:v) with ice-cold isotonic physiological saline, to determine antioxidant indices such as glutathione (GSH), malondialdehyde (MDA), catalase (CAT), glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD) (Total-SOD, CuZn-SOD and SOD-Mn) in serum, using assay kits for antioxidant indices purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) [14].
Total Immunoglobulins and their Fractions
Three samples of serum from each group were used to determine serum total immunoglobulins and their fractions (TIg, IgG, IgM and IgA) were detected by using ELISA kits, according to the instructions of Anjing Jiancheng Bioengineering Institute, Nanjing, China [15].
Statistical Analysis:
Data were summarized using Microsoft® Excel 2010 (10.2614.2625) Microsoft Egypt. The general liner model (GLM) was applied to test the differences among the five experimental groups. P-values less than 0.05 were considered to be statistically significant [16]. The statistical analysis was calculated using the following equation:
Yij = μ +Ti + Eij
Where:
Yij = Experiment observations.
μ = The overall mean.
Ti= The effect of dietary treatment.
i= T1, ----- T5.
Eij = The experimental error.
Duncan’s test was used to examine the significance degrees among means [17].
Results and Discussion
Antioxidant Enzymes
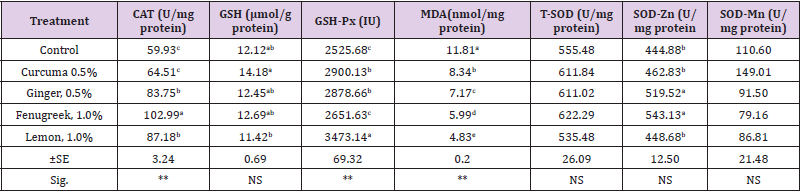
Data of antioxidant enzymes of dietary treatments are presented in (Table 2). The results revealed that there were significant differences (p≤0.01) among all dietary treatments on the values of catalase (CAT), glutathione peroxidase (GSH-Px) and Malondialdehyde (MDA). However, there were no significant differences (p>0.05) among all dietary treatments on glutathione (GSH) and Hepatic Superoxide dismutase (SOD).The present study demonstrated that rabbits fed dietary fenugreek seeds increased (p≤0.01) CAT, followed by those fed on lemon. Hence, rabbits fed dietary lemon recorded the greatest improvement (p≤0.01) in GSHPx followed by curcuma compared with other groups. The lowest value (p≤0.01) in MDA was recorded for rabbits fed lemon followed by fenugreek compared with other groups. Spices and herbs can have many benefits for the health of broilers and functions such as anti- oxidation ability [18], antimicrobial activity [19], enhance digestion by stimulating endogenous enzymes to the Brugalli at al. [20]. Ginger is widely used in many countries as a food spice and as an herbal remedy used [21]. Among the 81 chemical elements found in mammalian bodies, at least 19 are found in Citrus plants, including calcium (Ca), phosphorus (P), magnesium (Mg), potassium (K), sulfur (S), sodium (Na), iron (Fe), manganese (Mn), nickel (Ni), boron (B), silicon (Si), copper (Cu), zinc (Zn), molybdenum (Mo), selenium (Se), cobalt (Co), chromium (Cr), germanium (Ge) and arsenic (As) [22]. Of these elements, Mn, Fe, Cu, Zn and Se have been reported to be related to the antioxidant activity of organisms [23]. For example, Se, an essential component of antioxidant enzyme GSH-Px, can destroy free radicals in the cytoplasm and protect the tissues against oxidative damage [24]. Over 60 flavonoids have been found in citrus. Flavonoids have a direct role in scavenging reactive oxygen species, which can counteract lipid oxidation in vitro and improve the body’s antioxidant enzyme activity and decrease peroxide formation in vivo [25].
Table 2: Effect of natural feed additives on antioxidant enzymes.
a-eValues within the same column with different superscripts are significantly different (p≤0.05).SE:- standard error (±). NS: - Not significant. (**):- highly significant (p≤0.01).catalase (CAT), glutathione (GSH), glutathione peroxidase (GSH-Px), Malondialdehyde (MDA) and Hepatic Superoxide dismutase (SOD).
Decreasing lipid peroxidation by ginger treatment may be attributed to its antioxidant activity as it contains many phenolic compounds which have inhibitory effect on lipid peroxidation, these phenolic antioxidants may conserve the antioxidant enzymes but increase SH- containing compounds including glutathione. The depletion of antioxidant enzymes may be explained as ginger offered protection to cells against oxidative stress by scavenging free radicals [26]. This may be due to the presence of many antioxidative compounds like gingerols, shogaols, phenolic ketone derivatives, volatile oils and flavonoids in ginger, these antioxidant compounds may modulate spare the antioxidant enzymes [27]. These results are harmony with Zhang, et al. [28] showed that supplementation of ginger at the rate of 5g/kg significantly increased the activities of SOD and GSHPx and reduced MDA in broilers at the age of 21 and 42 days. The reduced level of MDA indicated that the addition of ginger alleviated the lipid peroxidative damage to the cell.
Humeral Immune Response (Immunoglobulin):
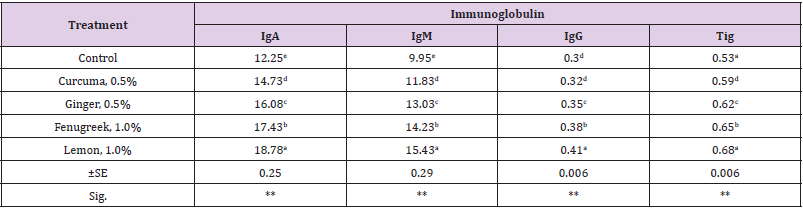
Data of immunoglobulin i.e. total immunoglobulin (Tig), immunoglobulin G (IgG), immunoglobulin M (IgM) and immunoglobulin A (IgA) of dietary treatments are presented in (Table 3). The results revealed that there was significant difference (p≤0.01) in all previous parameters among all dietary treatments. Rabbits fed dietary lemon recorded the highest (p≤0.01) value of Tig, IgG and IgM followed by fed on fenugreek seeds. However, the best (p≤0.01) IgA values were recorded for rabbits fed dietary lemon and control diet compared with other dietary treatments. Rabbits fed control diet recorded the lowest values of Tig and IgG compared with supplementaed diets. The improvement in immunoglobulin values as a result of adding feed supplementation to growing rabbits may be due to that herbal plants are rich in flavonoids such as garlic and turmeric extend the activity of vitamin C, act as antioxidants, and may therefore improve immune functions [29]. Moreover, Citrus pulp contain flavonoids and vitamin C which can present antioxidant properties [30,31], antibacterial [32] and immune stimulating activities [33-35].
Table 3: Effect of natural feed additives on immunoglobulin and their fraction of rabbits.
a-eValues within the same column have different superscripts are significantly different (p≤0.05).SE:- standard error (±). (**):- highly significant (p≤0.01).Total immunoglobulin (Tig), immunoglobulin G (IgG), immunoglobulin M (IgM) and immunoglobulin A (IgA).
References
- Attia YA, El Hanoun AM, Bovera F, Monastra G, El Tahawy, et al. (2014) Growth performance, carcass quality, biochemical and haematological traits and immune response of growing rabbits as affected by different growth promoters. J Anim Physiol Anim Nutr 98(1): 128-139.
- Peric L, Zikic D, Lukic M (2009) Application of alternative growth promoters in broiler production. Biotech Anim Husb 25(5-6): 387-397.
- Dalle Zotte A, Celia C, Szendrő ZS (2016) Herbs and spices inclusion as feed stuff or additive in growing rabbit diets and as additive in rabbit meat: a review. Livest Sci 189: 82-90.
- Upadhyay A, Upadhyaya I, Kollanoor Johny A, Venkitanarayanan K (2014) Combating pathogenic microorganisms using plant-derived antimicrobials: a mini review of the mechanistic basis. J Biomed Biotechnol p. 1-18.
- Alimohamadi K, Taherpour K, Ghasemi HA, Fatahnia F (2014) Comparative effects of using black seed (Nigella sativa) cuminseed (Cuminum cyminum) probiotic or prebiotic on growth performance blood haematology and serum biochemistry of broiler chicks. J Anim Physiol Anim Nutr 98(3): 538-546.
- Khatab AE, Hashem NM, El Kodary LM, Lotfy FM, Hassan GA (2016) Evaluation of the effects of cypermethrin on female reproductive function by using rabbit model and of the protective role of Chinese propolis. Biomed Environ Sci 29(10): 762-766.
- Hashem NM, Abd El Hady A, Hassan O (2013) Effect of vitamin E or propolis supplementation on semen quality, oxidative status and hemato-biochemical changes of rabbit bucks during hot season. Livest Sci 157: 520-526.
- Khalafalla MM, Abdellatef E, Dafalla HM, Nassrallah AA, Aboul Enein KM, et al. (2010) Active principle from Moringa oleifera Lam leaves effective against two leukemias and a hepatocarcinoma. Afr J Biotechnol 9(49): 8467-8471.
- Fimognari C, Turrini E, Ferruzzi L, Lenzi M, Hrelia P (2012) Natural isothiocyanates, genotoxic potential versus chemoprevention. Mutat Res 750(2): 107-131.
- El Desoky NI, Hashem NM, Elkomy A, Abo Elezz ZR (2017) Physiological response and semen quality of rabbit bucks supplemented with Moringa leaves ethanolic extract during summer season. Animal 11(9): 1549-1557.
- Ewuola EO, Sokunbi OA, Sanni KM, Oyedemi OM, Lawal TT (2015) Haematological and serum biochemical responses of rabbit does to crude Moringa oleifera leaf extract at gestation and lactation. Trop Anim Health Prod 47(4): 637-642.
- (2004) National Research Council (NRC), Nutrient Requirements of Poultry (9th). Natl Res. Counc, Natl Acad Press, Washington DC, USA.
- AOAC (2000) Official Methods of Analysis. In Gaithersburg Md (eds.) (17th). Association of Official Analytical Chemists, Arlington, VA.
- Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R (2015) Clinical relevance of biomarkers of oxidative stress. Antioxidants and redox signaling 23(14): 1144-1170.
- Wang W, Singh S, Zeng DL, King K, Nema S (2007) Antibody structure, instability, and formulation. Journal of pharmaceutical sciences 96(1): 1-26.
- SAS Institute (2003) SAS User‟s Guide: Statistics. SAS Institute, Cary, NC.
- Duncan DB (1955) Multiple ranges and multiple F-tests. Biometric 11: 1-42.
- Hui YH (1996) Oleoresins and essential oils. In: Hui YH (eds.). Bailey's industrial oil and fat products, Wiley-Interscience Publication, New York: pp. 145-153.
- Dorman HJD, Deans SG (2000) Antimicrobial Martins AP L Salgueiro MJ Goncalves AP da agents from plants: Antibacterial activity of plant volatile oils. J Appl Microbiol 88: 308-316.
- Brugalli I (2003) Alimentacao alternativa: a utilizacao de fitoterapicos ounutraceuticos como moduladores da imunidade e desempenho animal. Anais Do Simposio Sobre Manejo E Nutricao De Aves E Sunos Sao Paulo: Campinas: 167-182.
- Chrubasik S, Pittler M, Roufogalis B (2005) Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 12(9): 684-701.
- Zhou ZQ (2012) Citrus fruits nutrition. Science Press, Beijing, China.
- Amitava D, Kimberly K (2014) Antioxidant vitamins and minerals. Antioxidants in Food, Vitamins and Supplements, (5th) Chapter (15): pp.277-294.
- Levander OA, Ager AL, Beck MA (1995) Vitamin E and selenium: contrasting and interacting nutritional determinants of host resistance to parasitic and viral infections. Proceedings of the Nutrition Society 54(2): 475-487.
- Nakao K, Murata K, Itoh K, Hanamoto Y, Masuda M, et al. (2011) Anti-hyperuricemia effects of extracts of immature Citrus unshiu fruit. Journal of Traditional Medicines 28(1): 10-15.
- Saeidnia S, Abdollahi M (2013) Toxicological and pharmacological concerns on oxidative stress and related diseases. Toxicology and applied pharmacology 273(3): 442-455.
- Young HY, Luo YL, Cheng HY, Hsieh WC, Liao JC, et al. (2005) Analgesic and anti-inflammatory activities of [6]-gingerol. Journal of ethnopharmacology 96(1-2): 207-210.
- Zhang GF, Yang ZB, Wang Y, Yang WR, Jiang SZ, et al. (2009) Effects of ginger root (Zingiber officinale) processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. Poultry science 88(10): 2159-2166.
- Acamovic T, Brooker JD (2005) Biochemistry of plant secondary metabolites and their effects in animals. Proceedings of the Nutrition Society 64(3): 403-412.
- Santos GT, Lima LS, Schogor ALB, Romero JV, De Marchi FE, et al. (2014) Citrus pulp as a dietary source of antioxidants for lactating Holstein cows fed highly polyunsaturated fatty acid diets. Asian-Australasian journal of animal sciences 27(8): 1104-1113.
- Williams RJ, Spencer JP, Rice Evans C (2004) Flavonoids: antioxidants or signalling molecules? Free radical biology and medicine 36(7): 838-849.
- Nordi ECP, Costa RLD, David CMG, Parren GAE, Freitas ACB, et al. (2014) Supplementation of moist and dehydrated citrus pulp in the diets of sheep artificially and naturally infected with gastrointestinal nematodes on the parasitological parameters and performance. Veterinary parasitology 205(3-4): 532-539.
- Lee SJ, Shin JH, Sung NJ (2010) Comparison of physicochemical properties of Citron (citrus junos sieb ex tanaka) from three different areas of Namhae. J Agr Life Sci 44(5): 81-90.
- Ebrahimi A, Santini A, Alise M, Pourhossein Z, Miraalami N, et al. (2015) Effect of dried Citrus sinensis peel on gastrointestinal microbiota and immune system traits of broiler chickens. Italian Journal Animal Science 14(4): 712- 717.
- Pourhossein Z, Qotbi AAA, Seidavi A, Laudadio V CG, Tufarelli V (2015) Effect of different levels of dietary sweet orange (Citrus sinensis) peel extract on humoral immune system responses in broiler chickens. Anim Sci J 86(1): 105-110.

 Research Article
Research Article