Abstract
Objective: Bisphenol A [BPA] has been using throughout the world for years and makes a contribution to endocrine disruptor by oxidative stress induction in ovarian tissue. This study aimed to determine the effects of Ginger extract [GE], as an efficient antioxidant, on ovarian tissue in BPA-treated mice.
Materials and Methods: adult female mice were divided into 4 groups [n=6]: control, BPA [240mg/kg/day], GE [500 mg/kg/day] and BPA+GE. Oral treatment of GE was performed during 30 days. After treatments, to histological evaluation, ovaries were removed and processed. Serum MDA levels were also determined by the spectrophotometric method. Data were analyzed with One Way ANOVA test and P<0.05 was considered as statistically significant.
Results: The total volume of the ovary, cortex, medulla, Corpus luteum and the mean number of primordial, primary, preantral and antral follicles significantly decreased in the BPA groups compared with the control group [p<0.001]. A significant decrease in the volume of oocyte and its nuclei in the different types of follicles and also thickness of zona pellucida in preantral and antral follicles were observed in BPA group compared to control group [p<0.001]. Serum malondialdehyde [MDA] levels significantly increased in the BPA group compared with the control group [p<0.001]. Co-administration of GE and BPA compensated for the adverse effects of BPA on the above parameters. Furthermore, the mean number of different types of follicles significantly increased in GE-treated mice compared to the control group [p<0.001].
Conclusion: In the writer’s view, based on results, GE contributes significantly to improve the adverse effects of BPA in ovarian tissue
Keywords: Bisphenol A, Ginger extract, Ovary, Toxin
Introduction
Since the last decades, there have been numerous concerns regarding the prevalence of reproductive abnormalities in people. According to the previous studies, the incidence of reproductive abnormalities happens due to some environmental pollutants effecting on the endocrine system of mammals. They also believe these environmental pollutions disrupt the endocrine system [1]. These chemical constituents act like estrogens or they show anti-androgen activity. They employ this method to influence reproductive hormone-dependent activities. These pollutions can enter into the body in various ways which can be oral or respiratory [2].
BPA is one of the largest volumes of chemicals compound which has been producing. It will be produced more than six billion pounds annually [3]. Also, it is an external estrogen which pollutes both water and air. This chemical constituent is used in large scale in the industry. As a polycarbonate plastic monomer, BPA is a component of epoxy resin and polystyrene. It is also utilized in the production of various types of equipment and materials, such as dental filling material, disposable containers, the lining of cans, bottles of mineral water, children’s bottles of milk, appliances, toys, and household materials. Moreover, it can be utilized in structures of some polycarbonate and polymer additives as a softening substance [4].
It has been reported that BPA causes depletion of the antioxidant system making stress oxidative. During the studies, this constituent causes shaping hydroxyl free radicals and increases of hydrogen peroxide in the cells [5]. Besides, when the cell is exposed to BPA, the production of free radicals from the electron transport chain in mitochondria is highly increased [6]. According to the studies, BPA causes deactivation and reduction of antioxidant enzymes, such as superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and it finally produces oxidative stress [7]. Also, this substance leads to apoptosis and imposes oxidative stress through increasing the concentration of intracellular calcium, production of reactive oxygen species, and activation of caspase. Simply put these above-mentioned features make BPA as the main factor of damaged DNA [8].
BPA causes steroid inhibition, a decrease of progesterone, androstenedione, estradiol and disorder precursor in the synthesis of steroids, reducing the estrous cycle, and ovarian weight loss. This constituent also leads to having ovarian cysts that have a large number, a lesser extent corpus luteum, and many more follicle atresia [9]. Other harmful and pernicious effects of BPA are inhibition of follicular development, reduction in the level of the epithelium, stroma, ovarian glands, and reduction of estrous cycle [10]. Conversely, ginger, a powerful antioxidant in eliminating the harmful effects of BPA on the ovary, is a molecule which is a molecule which has the capability of slowing down or preventing the oxidation of other molecules. Oxidation is a chemical reaction in which electrons are transferred from one material to the oxidizing agent. On one hand, the antioxidants cause a finishing chain of reactions through free intermediate radicals. On the other hand, the antioxidants make themselves oxidized to inhabit other oxidative reactions [11].
The plant phenolics are multi-functional. They, besides, to be used as regenerative and inert oxygen-free radicals, can cause oxidation-reduction [12]. Ginger can be used as a strong natural antioxidant due to having volatile and non-volatile antioxidant constituents in different parts especially in its rhizome [13]. Specific odour and its flavor are due to zingiberene mix, Shogoal, gingerols, and the volatile oils which constitute 3% of the weight of fresh ginger. Their antioxidant properties are related to zingiberene, shogaols, gingerols, and Paradol [14]. Because of these findings, this study was undertaken to determine the beneficial properties of ginger on the ovarian damages in mice.
Materials and Methods
Animals and Treatments
In the present study, the female mice [NMRI] were used. These animals were taken from Pasteur Institute in Tehran. The female mice were divided into 4 groups [n=6]: control, BPA [250mg/kg/ day] [15], GE [500mg/kg/day] [16], and BA+GE. The treatment was performed in an oral way through gavage for 30 days.
Tissue Preparation
After the treatment period [30 days], the mice were anaesthetized by diethyl ether. After that, in order to prove ovarian tissue, the ovaries were removed, and they were put in glasses containing freshly prepared Bruins fixative. For 24 hours, they were kept in laboratory temperature. After 24 hours, the ovaries processing, samples were blocked in paraffin blocks.
Morphometrical Study
After the tissue processing, blocking was performed by Isector method and IUR sections [17,18]. 5 and 20μm thick sections were provided by microtome and they were stained with Hematoxylin and Eosin [H&E].
The Volume of Ovary, Cortex, Medulla and Corpus Luteum [mm3]
To examine the provided samples, morphometrical methods were used. To calculate the total ovarian volume by Cavalieri method [17], first 5 μm thick section were transferred on the work table. Then randomly counting probe was superimposed on the image. After that, the hit spots were counted with an image of the ovary. In the next step, the total number of points superimposed in each section on the image [ΣP] and obtained. By having a thickness of the section [t] and are associated with each point a[p] and entering this information in the following formula the total volume of the ovary was calculated.
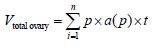
To get the volume of the ovary ingredient, the volume density for each was calculated as [17]:
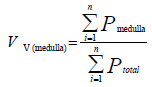
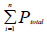 is the total number of counted points
is the total number of counted points
And 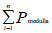 is the total number of points
is the total number of points
Superimposed on the medulla. The volume of medulla was then obtained through multiplying the volume density [Vv] by the total volume of the ovary.
The Volume of Oocyte, Follicular Cells and their Nuclei
To calculate the volume of oocyte and their nuclei the nucleator
method were used. 12 sections from 20μm thick sections was
randomly chosen and the selected follicles were studied using the
Olympus microscope with 100x magnification. To calculate the volume
of the oocyte, On the selected cells with an unbiased counting
frame, the distance from the center of nucleolus to the oocyte
membrane was measured [ ]. If the nucleolus were not clear, the
nucleus center was considered conventional. To estimate the volume
of oocyte nucleus the distance from the center of the nucleolus
to the nucleus membrane was measured. The measurements were performed in two different directions. The volume was calculated
by the following equation: 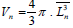 [19].
[19].
The Number of Follicles
To calculate the number of follicles, the optical dissector method
was used. The average 20μm thick sections were used. The sections
were studied using the Olympus microscope [BX41TE model] with
100x magnification and the Microcator [ND 221 B, Heidenhain,
Germany] connected to a computer. The nuclei of follicular cells
were sampled by an unbiased counting frame superimposed on
the monitor. According to the counting method on special frame
counter, those follicles are selected that their core is either inside
of the frame counter or on the lines. And they must be acceptable.
Those that are connected to forbidden lines are not acceptable and
they’re not counted. Then, the numerical density of follicles was
calculated according to the following equation [20]: 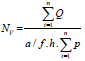
Σ Q is the total number of counted follicles
Σ p is the total number of the points superimposed on the selected fields is the tissue thickness considered for counting is the frame area in the true tissue scale. Then, the density number was multiplied by the total volume of the ovary to obtain the absolute number of follicles [21].
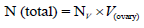
Zona Pellucida Thickness [μm]
To calculate the thickness of a zona pellucida [ZP], an average of 12 sections from 5-micron thick section was randomly selected. it has been taken photos out of a variety of selected follicles in different fields using a microscope with a photography camera [Olympus] that was made in Japan with Olysa software that was ob 100. To measure thickness, specific line grid [3 parallel lines] was randomly superimposed on the sampled fields. The crossing grid lines with a liner pellucid inner membrane area the tangent line with the inner membrane were considered [orthogonal intercept Method]. in brief by measuring the length of a line extended perpendicularly from the inner membrane to the outer surface of ZP at each intercept of the line of the grid with zona membrane and was considered as orthogonal intercept oi. The ovaries of mice were measured on average 100 to 200 hits. The average thickness of the zona pellucida was obtained from the following formula [22]:
Harmonic mean layer thickness = 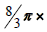 Harmonic mean
of orthogonal intercepts. Where Harmonic mean = number of
measurements / sum of the reciprocal of orthogonal intercept
lengths
Harmonic mean
of orthogonal intercepts. Where Harmonic mean = number of
measurements / sum of the reciprocal of orthogonal intercept
lengths
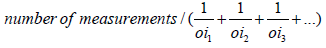
MDA Assay
First, a solution of TCA-TBA-HCl includes TCA [Trichloroacetic acid = TCA], TBARS [TBA] and HCl normal was prepared. Then, 1 ml of the samples with 2 ml of TCA-TBA-HCL solution were mixed and the samples were placed in the water boiling bath for 15 minutes. After removing the samples from the water bath, they were quickly cooled and centrifuged for 10 minutes. The supernatant was carefully separated and its absorption was at 532 nm to compare a blank containing all the ingredients except the sample. MDA concentration by using the extinction coefficient was calculated and expressed in micro molar [23].
Results
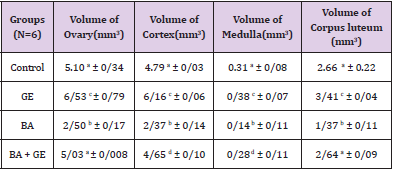
The Volume of Ovary, Cortex, Medulla and Corpus Luteum
The mean total volume of ovary, cortex, medulla and corpus luteum showed a highly significant reduction in BPA group compared to the others [p<0.01], while co-administration of BPA with GE increased the volume of ovary, cortex, medulla and corpus luteum to the control level (Table 1).
Table 1: Comparing the mean total volume of ovary, cortex, medulla and corpus luteum (mm3) in different groups of mice 30 days after treatment.
(Values are means ± SD. The means with the same code do not differ significantly (one- way ANOVA, Tukey test, p>0.05).
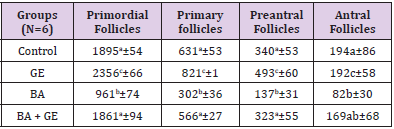
The Number of Follicles
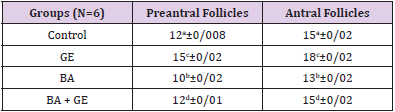
The mean number of the primordial follicles, primary, preantral, and antral follicles in the GE group to control group a significant increase was observed when compared to the other groups [p<0/001]. Also, in the BPA + GE group, reduction of the number of follicles in comparison to BPA group was in the control group was significantly compensated [p <0/001] (Table 2).
Table 2: Comparing the mean total number of follicles, the number of primordial, primary, preantral, antral follicles in different groups of mice 30 days after treatment.
(Values are means ± SD. The means with the same code do not differ significantly (one- way ANOVA, Tukey test, p>0.05).
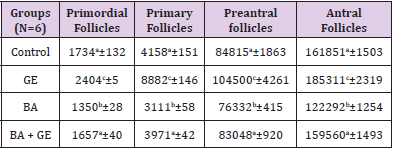
The Volume of Oocyte [μm3]
The mean volume of oocyte in primordial, primary, preantral, and antral follicles reduced significantly in BPA group compared to other groups [p<0.001], while in the BPA + GE group, GE compensated the reducing effect of BPA to the normal level [p<0.001]. The result also showed treatment with only GE caused a considerable increase in oocyte volume of the mentioned follicles [p<0.001]. (Table 3).
Table 3: Comparing the mean volume of oocyte (μm3) in different types of follicles in the groups of mice 30 days after treatment
(Values are means ± SD. The means with the same code do not differ significantly (one- way ANOVA, Tukey test, p>0.05).
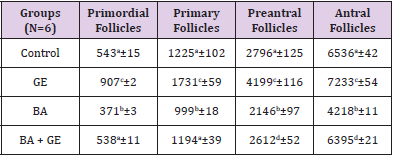
The Volume of Oocyte Nucleus [μm3]
The mean volume of the nucleus of the follicular cell in primordial, primary, preantral and antral follicles showed a highly significant reduction in the BPA group compared to the other groups [p<0.001]. The simultaneous treatment of mice with BPA + GE compensated the reduction of volume of follicular cell’s nucleus due to BPA in the above follicles. Meanwhile, the treatment of mice with only GE led to a highly significant increase in the mean volume of follicular cell’s nucleus in primordial, primary, preantral and antral follicles (p<0.001; Table 4).
Table 4: Comparing the mean volume of oocyte nucleus (μm3) in different types of follicles in the groups of rats 120 days after treatment. Values are means ± SD.
The Thickness of Zona Pellucida [μm]
Comparing the mean thickness of zona pellucida in the preantral and antral follicles a significant reduction was found in the BPA group [p<0.001], while co-administration treatment of mice with BPA + GE compensated these reductions [p<0.001] to the level of the control group (Table 5).
Table 5: Comparing the mean thickness of ZP (μm) in different groups of rats 120 days after treatment. Values are means ± SD.
(Values are means ± SD. The means with the same code do not differ significantly (one- way ANOVA, Tukey test, p>0.05).
MDA Serum Levels
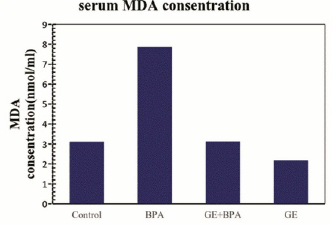
Comparing the MDA levels in the BPA group to the control group showed a significant increase [P <0/001]. Also, a significant reduction in the levels of MDA in the GE group compared to the control group was observed [P <0/001]. A meaningful change in the level of MDA in BPA + GE compared to the control group was not observed (P> 0.05; Figure 1).
Figure 1: Comparing the mean MDA serum levels in different groups of mice 30 days after treatment.
(Values are means ± SD. The means with the same code do not differ significantly (one- way ANOVA, Tukey test, p>0.05).
Discussion
This study revealed that BPA causes a reduction of the total volume of the ovary, cortex volume, medulla volume, and the corpus luteum volume. It also showed that the co_administration of BPA + GE can compensate for the destructive effects of BPA on the ovary. In the other words, BPA can cause destruction of corpus luteum and the number in the ovary [24], also BPA can change the level of estradiol, testosterone and progesterone hormones. It would be seen that it changes the GnRH secretion; this could be a reason for reducing the amount of corpus luteum and thereby reducing the size of the ovarian cortex and the total volume. estrogens such as estradiol stimulate the growth of follicles and protect follicles from atresia. Thus, the disturbance of estradiol production by BPA can lead to follicle atresia and reduced ovarian volume [10]. Free radicals play physiological and pathological roles during follicular, oocyte maturation, fertilization the corpus luteum. High accumulation of free radicals can cause ovarian apoptosis. BPA increase lipid peroxidation and the production of free radicals in the ovary [25]. The most important results of them are denaturation of proteins and the membrane lipid peroxidation, as well as increased permeability of the membrane.
These factors can cause the loss of cells that is probably due to the reduction of ovarian volume. These factors can cause the loss of cells that is probably due to the reduction of ovarian volume. GE accelerates the growth of ovarian follicles, increasing dens of connective tissue stroma, the rapid formation increasing secretion follicle and ultimately increasing the total volume of ovary, cortex and medulla [26]. Besides, the significant reduction of ovarian follicles, the oocyte volume and its nucleus in different follicles in BPA group compared to control group were observed, in which the detrimental effects had been compensated by co-administration of BPA + GE. BPA can cause steroidogenesis inhibition, a decrease of progesterone, androstenedione, estradiol and disturbance on the way of the synthesis of precursor steroids. Also, by disrupting the growth and development of follicles finally reduce them in their ovaries [27]. BPA can induce oxidative stress in the damages of ovarian tissue which results in decreasing of expression and disturbance in the expression of superoxide dismutase enzyme activity [the key antioxidant enzymes in the ovary] that it presumably makes an important factor in the destruction of follicles [7].
The analysis showed that BPA adversely affects the ovaries by increasing in production of reactive oxygen species by granulosa cells and inhibition of the growth of follicles [28] and finally reduces the number of them.
Also, BPA decreases oocyte volume and its nucleus by oxidative stress. BPA induce the apoptosis and inhibition of cell entry of the G2 phase of the cell cycle to M phase in the ovarian granulosa cells [29] leading to the inhibition of the growth and development of follicles. ginger due to having saponins can increase LH and FSH hormones from the pituitary and improving the process of folliculogenesis [30]. GE causes an increase in the activity of superoxide dismutase, glutathione reductase and Glutathione peroxidase. GE inhibits lipid peroxidation and oxidative stress in the ovary [31]. This would be a reason to compensate for the destructive effects Bisphenol on the ovarian and a follicular reduction that is compensated for due to it.
The following study also illustrated that Bisphenol can cause decreasing of the thickness of zona pellucida in preantral follicles and antral follicles that zona pellucid has consisted of glycoprotein, and as it mentioned earlier, BPA can decrease the level of proteins [32]; Therefore, the reduction of the thickness of zona pellucida in follicles is something normal. On one hand, BPA results in a reduction in estradiol production, in a way that it can reduce the proliferation of granulosa cells and showed an increase in apoptosis [27]. Bisphenol induces apoptosis in granulosa cells thus, BPA by the induction of apoptosis in granulosa cells, decreasing estradiol and LH in both direct and indirect ways causes a decrease of the thickness of zona pellucida. GE by stimulating gonadotropin secretion of LH and FSH hormones can cause the production of estrogen and by boosting antioxidant systems can compensate for the reduction of the thickness of zona pellucida in follicles [30].
Besides, the measuring of lipid peroxidation showed that BPA would increase the production of MDA. When the cell exposed to BPA, the production of free radicals will increase in electron transport chain into the mitochondria [6]. According to the study, BPA causes deactivation and decrease of antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase finally producing oxidative stress [31]. On the other side, GE due to having volatile and non-volatile antioxidant compounds especially in its different parts of the rhizome which can be used as a natural antioxidant, vitamin C and flavonoids and phenols contain in this extract can inhibit the lipid peroxidation. By boosting the antioxidant system and improving the performance of ovarian cells, ginger can compensate for the reduction of the thickness of zona pellucida in follicles. This extract also inhabits protein denaturation and membrane lipid peroxidation [26] Thus, it prevents the loss of proteins and glycoproteins and eventually pellucida thickness would have remained.
Conclusion
Based on the results of this study GE can noticeably improve the adverse effects of BPA on ovarian tissue disorders in adult mice. It can also be used because of the effective herbal antioxidants that have against toxicity oxidative stress caused by BPA on ovarian tissue.
References
- Lilja Nielsen, erik baatrup (2006) Quantitative studies on the effects of environmental estrogens on the testis of the guppy. Aquat Toxicol 80(2): 140-148.
- Lopez Jacki (2010) Endocrine-disrupting chemical pollution: why the EPA should regulate these chemicals under the clean water act. Sustainable Development Law and Policy 10(3): 19-22.
- Laura N Vandenberg, Russ Hauser, Michele Marcus, Nicolas Olea, Wade V Welshons (2007) Human exposure to bisphenol A (BPA). Reproductive toxicology 24(2): 139-177.
- Antonia M Calafat, Zsuzsanna Kuklenyik, John A Reidy, Samuel P Caudill, John Ekong, et al. (2005) Urinary Concentrations of Bisphenol A and 4-Nonylphenol in a Human Reference Population. Environ Health Perspec 113(4): 391-395.
- Natalie R Gassman (2017) Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ Mol Mutagen 58(2): 60-71.
- Somaira Khan, Saba Beigh, Bhushan P Chaudhari, Shikha Sharma, Sayed Aliul Hasan Abdi, et al. (2016) Mitochondrial dysfunction induced by Bisphenol A is a factor of its hepatotoxicity in rats. Environ Toxicol 31(12): 1922-1934.
- Kabuto H, Hasuike S, Minagawa N, Shishibori T (2003) Effects of bisphenol A on the metabolisms of active oxygen species in mouse tissues. Environ Res 93(1): 31-35.
- Yuan-Jie Li, Tian-Bao Song, Yan-Yan Cai, Xiao-Lin Wu (2009) Bisphenol A Exposure Induces Apoptosis and Upregulation of Fas/FasL and Caspase-3 Expression in the Testes of Mice. Toxicological Sciences 108(2): 427-436.
- Jackye Peretz, Zelieann R Craig, Jodi A Flaws (2012) Bisphenol A Inhibits Follicle Growth and Induces Atresia in Cultured Mouse Antral Follicles Independently of the Genomic Estrogenic Pathway. Biology of Reproduction 87(3): 1-11.
- Jackye Peretza, Jodi A Flaws (2013) Bisphenol A down-regulates rate-limiting Cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicol Appl Pharmacol 271(2): 249-256.
- Sies H (1997) Oxidative stress: oxidants and antioxidants. Experimenta Physiology 82(2): 291-295.
- Lan Su, Denys Charles, Jun-Jie Yin, Liangli Lucy Yu (2007) Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chemistry 100(3): 990-997.
- Villupanoor A Parthasarathy, Bhageerathy Chempakam, T John Zachariah (2008) Chemistry of Spices. Chapter 11.
- Fereshteh Dadfar, Seyed Ebrahim Hosseini, Aminollah Bahaoddini (2014) A review of phytochemical, pharmacological and physiological properties of ginger (zingiber officinale). Journal of Clinical Excellence 3(1): 72-86.
- Rehab Hussein, Jehane Ibrahim (2013) Pathological mechanisms of liver injury caused by oral administration of bisphenol A. Life Science Journal 10(1): 663-673.
- Morakinyo AO, Adeniyi OS, Arikawe AP (2008) Effects of Zingiber Officinale on Reproductive Functions in the Male Rat. African Journal of Biomedical Research 11(3): 329-334.
- Howard C, Reed M (1998) Unbiased stereology: three-dimentional measurement in microscopy. United Kingdom: Bios Scientific Publishers 194: 153-157.
- Mouton PR (2002) Principles and practices of unbiased stereology: An introduction for bioscientists. Baltimore and London: The Johns Hopkins University Press.
- Calado AM, Rocha E, Colaco A, Sousa M (2001) Stereologic characterization of bovine (Bos taurus) cumulus-oocyte complexes aspirated from small antral follicles during the diestrous phase. Biol Reprod 65: 1383-1391.
- Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB (2004) Methods for quantifying follicular numbers within the mouse ovary. Reproduction 127: 569-580.
- Wang Y, Newton H, Spaliviero JA, Allan CM, Marshan B, et al. (2005) Gonadotropin control of inhibin secretion and the relationship to follicle type and number in the hpg mouse. Biol Reprod 73: 610-618.
- Ferrando RE, Nyengaard JR, Hays SR, Fahy JV, Woodruff PG (2003) Applying stereology to measure thickness of the basement membrane zone in bronchial biopsy specimens. J Allergy Clin Immunol 112: 1243-1245.
- Lapenna D, Ciofani G, Pierdomenico SD, Giamberardino MA, Cuccurullo F (2001) Reaction conditions affecting the relationship between thiobarbituric acid reactivity and lipid peroxides in human plasma. Free Radic Biol Med 31(3): 331-335.
- Marina Fernández, Nadia Bourguignon, Victoria Lux-Lantos (2010) Neonatal Exposure to Bisphenol A and Reproductive and Endocrine Alterations Resembling the Polycystic Ovarian Syndrome in Adult Rats. Environ Health Perspect 118(9): 1217-1222.
- J Sravani, K Padmaja, P Eswara Prasad, B Punya Kuma (2016) Effect of Bisphenol-A on Antioxidant Enzymes and Lipid Peroxidation in Liver of Chick Embryos. International Journal of Meat Science 6(1): 1-5.
- Omar Abu Bake S (2013) Effect of Ginger on the Histological Structure of Some Organs of Female Rats and Their Embryos during Pregnancy. Life Science Journal 10(2): 1225-1232.
- Jackye Peretz, Rupesh K Gupta, Jeffrey Singh, Isabel Hernandez-Ochoa, Jodi A Flaw (2011) Bisphenol A Impairs Follicle Growth, Inhibits Steroidogenesis, and Downregulates Rate-Limiting Enzymes in the Estradiol Biosynthesis Pathway. Toxicological Sciences 119(1): 209-217.
- Quirk SM, Cowan RG, Harman RM, Hu CL, Porter DA (2004) Ovarian follicular growth and atresia: the relationship between cell proliferation and survival. J Anim Sci 82(13): 40-52.
- Xu J, Osuga Y, Yano T, Morita Y, Tang X, et al. (2002) Bisphenol A induces apoptosis and G2-toM arrest of ovarian granulosa cells. Biochem Biophys Res Commun 292(2): 456-462.
- Amir Mahdi Imani, Nava Ainehchi (2014) Comparison of the Effects of Methotrexate and Ginger Extract on Reproductive Parameters in Rats. C Medical and Biological Sciences 1(1): 103-109.
- Ramudu Kondeti, Kondeti ramudu Shanmugam, Mallikarjuna Korivi, K Hema Chandra Reddy (2011) Protective effect of dietary ginger on antioxidant enzymes and oxidative damage in experimental diabetic rat tissues. Food Chemistry 124(4): 1436-1442.
- Peerut Chienwichai, Supachai Topanurak, Onrapak Reamtong, Usa Boonyuen, Suwalee Worakhunpiset, et al. (2018) Effects of low bisphenol A concentration on protein expression profiles in an in vitro model of non-alcoholic fatty liver disease. Molecular & Cellular Toxicology 14(1): 61-70.

 Research Article
Research Article