Abstract
Introduction: Nowadays, cryopreservation of human embryos has become a required part of IVF programs. The purpose of this study was to compare the recovery, survival, cleavage and blastocyst development rate of pronuclear stage human embryos, after slow freezing and vitrification methods.
Material and Methods: Human 2PN stage embryos were divided randomly into four groups. In the 1M (control) group 29 embryos included without any exposure. In the sham or 2nd group 15 embryos were considered to survey for cryoprotectant toxicity. Embryos in the 3rd and 4th group freezed by vitrification and slow freezing methods respectively. Embryos in 3rd group (n=36), were vitrified using a new vitrification solution. Survived embryos were cultured, incubated and evaluated for 5 days post-thawing. In 4th group, 2PN embryos (n=41) were freezed in propanediol (PROH) and sucrose 16-18 hours pos- ICSI using a programmed freezing instrument (Planner Series III) and plunged into liquid nitrogen.
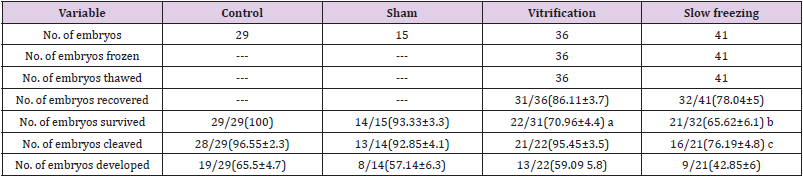
Results: Our data showed that survival rate in 1M (control) group was significantly (P<0.05) more than other groups, meanwhile cleavage rate in the control group is significantly more than only slow freezing (4th) group (96.55% vs 76=19%). The blastocyst formation rates in the 3rd and 4th groups are significantly lower than control group (59.09% and 42.85% vs 65.51%). Comparison of cleavage and blastocyst formation rate showed a significant difference between 3rd and 4th groups. The survival rate has a significant difference between groups (100%, 93.33%, 70.96% and 65.62% respectively) (P<0.05).
Discussion: The pronuclear stage embryos from ICSI could choosen for cryopreservation because the developmental capacity of the embryos, after thawing, can easily be ascertained and the predominance of study has shown that human embryos survive and implant at higher rates, when frozen in 2PN.
Conclusion: Both procedures had nearly similar effect on 2PN human embryos, but the vitrification method is better because of its simplicity, quickness and cost effectiveness.
Keywords: Human; Vitrification; Slow freezing; Development
Introduction
Human embryo cryopreservation is widely used in ART Programs, as it allows embryos to be stored without deterioration until needed [1]. Human pronuclear stage embryos (2PN) are routinely crupreserved by use of slow controled-rate freezing procedure. This method requires expensive equipments and longtime [2]. A simpler, quicker and less expensive alternative to crupreservation is vitrification [3,4]. The high concentration of cryoprotectant in the vitrification solution is so that when immersed into liquid nitrogen the solutions form a glass entity; this prevents the formation of extra-and /or intracellular ice crystals during cooling and thawing [2]. All known penetrating cryoprotectants are toxic at high concentrations [1,5-8]. Previous studies have indicated that addition of a nonpermeating agent, such as sucrose or polymer (for e.g. ficoll or dextran) to the mixture allows both intra-and extracellular vitrification at less toxic concentrations of permeating cryoprotectant agcents and improves survival rate [1,9-12].
It was shown that solutions containing 35% ficoll and 25% ethylene glycol used in vitrification cause high rate of blastocyst from 2-cell mouse embryos [1]. In cru preservation the embryos were chosen at 2PN stage because:
i. it permits the study of a uniform population of embryos at interphase of mitotic division. without any fragmnts which are known to influence cryopreservation [2,13],
ii. the post-thawing developmental capacity of the embryos, can easily be ascertained [2,14]and
iii. most of the study has shown that human embryos, when frozen in 2PN, survive and implant at higher rates in comparison with the cleavage stage [4,15-17]. Although a variety of more effective slow and rapid Cooling procedures have been developed for cow and mouse embryos, these established protocols are not necessarily effective for other species embryos [1]. It was therefore decided to examine the effectiveness of this new solution on the recovery, survival, cleavage and blastocyst formation rate of 2PN embryos postvitrification and comparing. The aim of this study was to introduce a cryopreserved method which not only had fewer negative effects on survival, cleavage and development but also was simpler, faster and more cost effective.
Materials and Methods
Cumulus oocyte complexes of patients underwent downregulation ovarian stimulation were collected by ultrasoundguided follicle aspiration 34-36 hours post hCG administration at Navid’s Institute of Infertility (Tehran, Iran). All the mature oocytes subjected to intracytoplasmic sperm injection (ICSI). The injected oocytes were placed in IVF-medium FertiPro, FP02 Fc01, and Belgium) and incubated at 37 °C and 5% CO2 nearly 16-20 hour’s post-ICSI. The probable embryos were observed by sterio microscope (Nikon, Japan) and inspected for the presence of two pronuclei (2PN). The high-quality embryos at 2PN stage were washed incubated and cultured in Gl medium for 48 hours and subsequently were transferred to patients. The supernumerary 2PN embryos of couples that both of them had signed the consent forms prior to treatment were selected for cryopreservation by Vitrification or slow-freezing method. The 2PN stage embryos which were qualitatively well were transferred to patient’s uterine tube and the excess embryos cryopreserved according to their features.
Vitrification Method
The vitrification of 2PN embryos performed according previously described method [1]. The sibling 2PN embryos of same patients were placed in PBS medium (at room temperature) for 5 minutes and then pre-equilibrated in PBS (supplemented with 25% EG) for 3 minutes. Briefly vitrification solution was prepared by dissolving 3.5gr ficoll (Sigma, F-2878). 2.5 ml of ethylene glycol (Sigma, E-9129) and 1.026 gr of sucrose (Sigma, S-7903) in PBS medium. The embryos were aspirated with minimal volume of solution into a finely drawn glass pipette and expelled to vitrification solution. preparation of the straws was done by aspiration of 5 cm of 0.5 M sucrose,1 cm of air bubble, and 3 cm of vitrification solution with embryos. After transferring the embryos to a straw, a second air bubble were aspirated until the cotton plug became wet subsequently the open end of the straw was hold up above the LN2 into 37 °C water bath for 1 minute. Then passed through 0.5 M, 0.3 M and 0.1 M of sucrose (thawing solutions) for 5, 3 and 3 minutes, respectively. Following washing up in PBS medium, the embryos were inspected for survival rate under an inverted microscope. The surviving embryos were incubated at 37 °C and 5% Co2 and cultured in IVF-medium up to 3 days, then transferred to G3-medium up to day 6.
Cryopreservation Protocol
The supernumerary 2PN embryos of each patient were cryopreserved if their tow pronuclei aligned at the equatorial plate, the cytoplasm was clear and had no fragmentation [14,15]. In order to avoid the intervening factors, sibling embryos of each patient were divided randomly into four groups. The embryos in the 1st (control) group (n=29) included without any exposure. In the sham 2nd group (n=15) embryos were considered to survey for cryoprotectant toxicity. Embryos in the 3rd (n=36) and 4th (n=41) group freezed by slow freezing or vitrification methods respectively. For slow-freezing procedure, 2PN stage embryos were incubated sequentially in each of the following for 10 minutes at room temperature: A: 1 ml of phosphate-buffered saline (PBS), B: 1.5 M of propanediol (PROH) in PBS medium, and C: 1.5 M of PROH and 0.1 M of sucrose in PBS medium (Freeze-KIT, 10012, Vitrolife, Goteborg). Then, embryos were loaded into 250 μl straws (Minitube, 1904/0010) and transferred to a biological programmed freezer chamber (KRYO 10 Planer Series III, UK) with a chamber of 22 °C temperature.
The temperature was lowered to –7 °C at a rate of –2 °C/min. After 5 minutes soak time at –7 °C, ice nucleation was induced manually by touching the straws with LN2–cooled forceps at the level of the fluid meniscus. The embryos were hold at –7 °C for another 5 minutes post according. In this way the temperature was reduced to –30 °C at a rate of –0.3 °C/min. The straws were cooled furtherly by cooling rate of –50 °C/min up to –140 °C and help in this temperature for 1 minute. Finally, the straw was plunged into liquid nitrogen [18,19]. After straws storing in LN2-containers for 1 month, thawing was done by keeping them at room temperature for 40 seconds, then immersed in 37 °C warm water bath for 40 seconds, until ice crystals disappeared in the medium. Removal of the cryoprotectants was done in four steps for 10, 10, 5 and 5 minutes sequentially as follow: A) 1 M PROH +0.2 M sucrose in PBS medium, B) 0.5 PROH +0.2 M sucrose, C) 0.2 M sucrose and D) PBS medium alone. All steps were done at room temperature (Thaw- KIT, 10013, Vitrolife, coteborg). After checking up, the survived 2PN embryos were incubated at 37 °C and 5% Co2 in IVF-medium for 3 days and then transferred to G2-medium (FertiPro, FP0IFG04, Belgium) for another 2 days.
Assessment of Thawed Embryos
After thawing, the embryos recovery, survival, cleavage and blastocyst formation rate of frozen-thawed pronuclear stage embryos were evaluated. Recovery of 2PN embryos was calculated as the ratio of embryos observed under the microscope after thawing to the initial number of frozen embryos. Surviving embryos were defined as the percentage of recovered embryos with morphologically intact clear cytoplasm which could be returned to their original isotonic volume after thawing and subsequent dilution of cryoprotectant. The cleavage rate was defined as the percentage of surviving embryos that cleaved to two cells or more, after 24 hours. Blastocyst developmental rate (BDR) was defined as the percentage of surviving blastocyst stage embryos related to original number of freezed embryos. To reduce the rate of stickness, during vitrification, straws were washed with 0.5 M sucrose.
Assessment of Toxicity of Vitrification Solution for 2PN Embryos (sham or 2nd group)
Randomly selected supernumerary 2PN stage embryos (n=15) were evaluated after treatment with vitrification solutions (without freezing); PBS medium (for 5 minutes), 25% EG (for 3 minutes) and vitrification solution (for 30 seconds). All steps were done at room temperature. The removal of cryoprotectant agents was similar to removal of cryoprotectant agents in vitrification method. Then, the survival rate, cleavage and BDR were evaluated according to the other groups.
Statistical Analysis
Statistical analysis was done by using X² test. P values less than 0.05 were considered as significant.
Results
In this study a total number of 121 Pronuclear stage embryos were used and divided into control or non- frozen, (n=29), sham (n=15), 1st experimental (vitrification, n=36) and 2nd experimental (slow-freezing, n=41) groups (Table 1). In control group, the mean percent of survival, cleavage and developmental rate were 100, 96.55 and 65.51 percent, respectively. Assessment of 2PN embryos that were only exposed to vitrification solutions (sham group) showed that the survival, cleavage, and development to blastocyst had no significant difference (P>0.05) between groups (Table 1). The number of post-thawing recovered embryos in vitrification 31 (86.11%) and slow freezing 32(78.04%) groups have significant difference (P<0.05). The survival, cleavage and blastocyst formation rate in the 3rd and 4th expermental groups were shown in Table 1. Comparison of survival rate between control (1st) and slow freezed (4th) groups showed that there was a significant difference between groups, similarly the difference between cleavage and blastocyst formation rate in these groups was significant. Comparison of survival rate between non-frozen vitrified (2nd or sham) and vitrified (3rd) groups showed that the difference between them was significant, but the cleavage and developmental rate was not significant (P<0.05) (Table 1). This study showed that there has a non-significant difference for survival rate between vitrification (3rd) and slow freezing (4th) groups while the cleavage and development rate have significant difference (P<0.05) (Table 1).
a. Significant difference of survival rate between control and slow freezing groups (P<0.05).
b. Significant difference of survival rate between control and vitrification groups (P<0.05).
c. Significant difference of cleavage rate between control and vitrification groups (P<0.05).
All number in the parenthesis shows the mean percent ± standard deviation.
Discussion
This study shows that vitrification of human 2PN stage embryos could enhance the cleavage and development of embryos post thawing related to slow freezing methods. The solutions used for cryopreservation had no toxic effects on human 2PN embryos. The survival rate of 2PN stage embryo was higher in vitrification method compare with slow freezing method but their differences was not significant. Nowadays, cryopreservation of 2PN stage embryos by vitrification or slow-freezing methods have very little practical impact on 2PN stage embryos. Jelinkova et al reported a postthawing twin pregnancy from vitrified 2PN stage embryos using ethylene glycol (EG) and trehalose. Their method [3] was a safe and promising for cryopreservation of human zygotes although its method was different with the present study the result was similar. Recent studies demonstrated successful result after vitrification of human oocytes, cleaved embryos and blastocysts with solutions containing polymers [3,20,21]. The vitrification solution containing 25% EG, 35% Ficoll, was not toxic for 2-8 cell stage mouse embryos, developed to blastocyst [1].
Rapid Cooling and vitrification protocols are particularly attractive cryopreservation strategies for embryos as they are cheap, fast, and simple, but most are embryo toxic as the solutions contain high cryoprotectant concentrations. Considerable effort is therefore still being directed toward improvement of rapid Cooling and vitrification protocols for embryos. Results showed that some embryos have been lost, after thawing. Higher number of 2PN embryos was recovered, after slow freezing in compare with vitrification method, although the difference was not significant statistically. The lost embryos were attributed to embryos sticking to the straw wall in both methods [1]. During vitrification procedure, modified solution caused higher number of embryos stick to the straw wall due to high osmolality. Another reason for losing the embryos using vitrification method, was rapid thawing, which prevents ice crystal formation. Embryo entering to LN2 during storage and very rapid thawing, causes explosion of the straws while transferring to warm water bath, thus, it decreases the rate of embryo recovery. These studies show that explosion may be avoided by holding straws in LN2 vapor for a longer period, before immersing it to warm water bath [2,22].
The survival rate on control, vitrified and slow freezing groups showed a significant difference between groups (100%, 70.96%, 65.62%). The reason for lower survival rate of slowfrozen 2PN embryos comparing with control group is extra-cellular ice formation during cooling that causes trapping of embryos between ice crystals and damaging the zonae pellucid [23]. The number of surviving 2PN embryo does not corelate to the use of vitrification solutions, because comparison of sham and control groups has demonstrated that the solutions have not any toxic effect on survival, cleavage and blastocyst formation rate, probably because oocyte and zygotes are more sensitive to osmotic shock, and they have lower permeability to cryoprotective additives [3,24]. Additionally, vitrified cells are more sensitive to hypotonic stress than fresh cells in post-warming duration [25]. To reduce the hypoosmotic stress, non-permeating compounds are used as osmotic buffers during removal of permeating cryoprotectant from inside the cells [25]. Breaking of the vitrification solution (during cooling) is another reason for damaging the embryos and reducing the embryos survival rate finally [26], which is in agreement with Liebermann et al, that decrease in survival rate after vitrification is itself a problem [27].
The non-significant difference of cleavage and BDR between non-frozen and slow controlled groups for frozen embryos showed that the surviving pronuclear stage embryos (after thawing) could act similar to the fresh embryos. The cleavage rate of vitrified embryos was significantly lower than control group (P<0.05), but the cleaved embryos had similar potential for development in comparing with fresh embryos. When 2PN embryos with best morphological quality were transferred to the patients and supernumerary embryos were cryopreserved, development of 2PN embryos cultured in vitro was higher than the frozen one. Previous studies showed that there is a relationship between morphology of the zygotes with its ability to continue development, both in vivo and in vitro at 16-18 hours post insemination [2,23,28,29] which is in concordant with the-our finding in the present study. Studies of Kuleshova and, Abbeel showed that during rapid Cooling procedures, number of surviving embryos with a sustained capacity for further cleavage is depended on an optimal exposure time for cryoprotectant solution before freezing.
This finding showed that permeating of the penetrating cryoprotectant is very important [1,2]. For human pronuclear stage embryos, neither the optimal concentration of cryoprotective agent, (especially concentration of polymers in vitrification solution), nor the optimal time of exposure to the vitrification solutions is not known. Permeability characteristics of human pronuclear embryos towards vitrification solution are not known and may have some quantitatively difference with mice embryo. Sub-optimal exposure time of the pronuclear embryos to the pre-equilibration (25%EG) and vitrification solutions may lead to insufficient permeation of the ethylene glycol, and hence sub-optimal results. Our results in the present study clearly demonstrated that, after a randomized comparison, vitrification of 2PN embryos with modified solution 25EG: 35F has similar efficiency as slow freezing. It is proposing that before using this new solution for cryopreservation of human pronuclear stage embryos, their reproducibility and safety should be studied.
Conflict of interest
There is no conflict of interest between authors and none of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Acknowledgments
We thank all members of our IVF team who participated in collecting zygotes.
References
- Kuleshova LL, shaw JM, Trounson AO (2001) Studies on replacing most of the penetrating cryoprotectant by polymers for embryo cryopreservation. Cryobiology 43(1): 21-31.
- Van den Abbeel E, Camus M, Van Waesberghe L, Devroey P, Van Steirteghem Ac, et al. (1997) A randomized Comparison of the cryopreservation of one-cell human embryos with a slow controlled rate cooling procedure or a rapid Cooling procedure by direct plunging into liquid nitrogen. Hum Rcprod 12(7): 1554-1560.
- Jelinkova L, Selman HA, Arva A, Strehler E, Reeka N, et al. (2002) Twin pregnancy after vitrification of 2-pronuclei human embryos. Fertil Steril 77(2): 412-414.
- Demoulin A, Jouan C, Gerday C, Dubois M (1991) Pregnancy rates after transfer of embryos obtained from different stimulation protocols and frozen at either pronucleate of multicellular stages. Hum Reprod 6: 799-804.
- Kasai M, Nishimori M, Zhu SE, Sakurai T, Machida T, et al. (1992) Survival of mouse morula vitrified in an ethylene glycol-based solution after exposure to the solution at various temperatures. Biol Reprod 47(6): 1134-1139.
- Mukaida T, Wada S, Takahashi K, Pedro PB, An TZ, et al. (1998) Vitrification of human embryos based on the assessment of suitable conditions for 8-cell mouse embryos. Hum Reprod 13(10): 2874-2879.
- Rall WF, Fahy GM (1985) Ice-free cryopreservation of mouse embryos at 196 °C by vitrification. Nature 313(6003): 573-575.
- Rall WF, Wood MJ, Kirby C, Whitting DG (1987) Development of mouse embryos cryopreserved by vitrification. J Reprod Fertil 80(2): 499-504.
- Kasai M, Komi JH, Takakamo A, Tsudera H, Sakurai T, et al. (1990) A simple method for mouse embryo crupreservation in a low toxicity vitrification solution, without appreciable loss of viability. J Reprod Fertil 89(1): 91-97.
- Libo SP, Oda K (1993) High survival of mouse zygotes and embryo cooled rapidly or slowly in ethylene glycol plus polyvinylpyrrolidone. Cryo-Letters 14: 133-144.
- Saha S, Otoi T, Takagi M, Boediono A, Sumantri C, et al. (1996) Normal calves obtained after direct transfer of vitrified bovine embryos using ethylene glycol, trehalose, and Polyvinylpyrrolidone. Cryobiology 33(3): 291-299.
- Friedler S, Giudice LC, Lamb EJ (1988) Cryopreservation of embryos and ova. Fertil Steril 49(5): 743-762.
- Camus M, Van den Abbeel E, van Waesberghe L, Wisanto A, Devroey P, et al. (1989) Human embryo viability after freezing with dimethylsulfoxide as a cryoprotectant. Fertil Steril 51(3): 460-464.
- Damario MA, Hammitt DG, Galanits TM, Session DR, Dumesic DA, et al. (1999) Pronuclear stage cryopreservation after intracytoplasmic sperm injection and conventional IVF: implications for timing of the freeze. Fertil Steril 72(6): 1049-1054.
- Damario MA, Hammitt DC, Session DR, Dumesic DA (2000) Embryo crupreservation at the pronuclear stage and efficient embryo use optimizes the chance for a liveborn infant from a single oocyte retrieval. Fertil Steril 73: 767-773.
- Quinn P (1990) Success of oocyte and embryo freezing and its effect on outcome with in vitro Semin Reprod Endocrinol 8(4): 272-280.
- Veeck LL, Amundson CH, Brothman LJ, Desceisciolo C, Maloney MK, et al. (1993) Significantly enhanced pregnancy rates per cycle through cryopreservation and thaw of pronuclear stage oocytes. Fertil Steril 59(6): 1202-1207.
- Trounson AO (1990) Cryopreservation. British Medical Bulletin 46: 695-708.
- Macas E, Imthurm B, Borsos M, Rosselli M, Maurer Major E, et al. (1998) Impairment of the developmental potential of frozen-thawed human zygotes obtained after intracytoplasmic sperm injection. Fertil Steril 69(4): 630-635.
- Lane M, Schoolcraft WB, Gardner DK (1999) Vitrification of mouse and human blastocysts using a novel cryoloop container-less technique. Fertil Steril 72(6): 1073-1078.
- Shaw JM, Kuleshova LL, MacFarlane DR, Trounson AO (1997) Vitrification properties of solution of ethylene glycol in salin containing PVP, Ficoll, or dextran. Cryobiology 35(3): 219-229.
- Rall WF, Wood MJ (1994) High in vitro survival of day 3 mouse embryos vitrified of frozen in a non-toxic solution of glycerol and albumin. J Reprod Fertil 101(3): 681-688.
- Balakier H, MakLusky NJ, Casper RF (1993) Characterization of the first cell cycle in human zygotes: implications for cryopreservation. Fertil Steril 59: 359-365.
- McWillams RB, Gibbons WF, Leibo SP (1995) Osmotic and physiological responses of mouse zygotes and human oocytes to mono and disacharides. Hum Reprod 10(5): 1163-1171.
- Pedro PB, Zhu SE, Makino N, Sakurai T, Edashige K, et al. (1997) Effects of hypotonic stress on the survival of mouse oocytes and embryos at various stages. Cryobiology 35(2): 150-158.
- Rall WF (1987) Factors effecting the survival of mouse embryos cryopreserved by vitrification. Cryobiology 24(5): 387-402.
- Liebermann J, Tucker MJ (2004) Vitrifying and warming of human oocytes, embryos, and blastocysts: vitrification procedures as an alternative to conventional cryopreservation. Methods Mol Biol 254: 345-364.
- Scott L, Alvero R, Leondires MM, Miller B (2000) The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod 15(11): 2394-2403.
- Wright G, Wiker S, Elsner C, Kort H, Massey J, et al. (1990) Observations on the morphology of pronuclei and nucleoli in human zygotes and implications for cryopreservation. Hum Reprod 5(1): 109-115.

 Research Article
Research Article