Abstract
Objective: The purpose of this study was to evaluate the effect of J-Plasma® and the argon beam coagulator (ABC) on porcine bowel.
Methods: J-Plasma® (test) and the Argon Beam Coagulator (control) were used to treat the jejunum, ileum, and large intestine of seven (7) female Yorkshire cross pigs (J-Plasma: acute, n=1 and chronic n=3; ABC: acute n=1 and chronic n=2). The acute animals were euthanized 4 hours following treatment and the chronic animals were survived to 14 days then euthanized. Following euthanasia, all treated sites were evaluated grossly for evidence of perforation.
Results: Perforations were observed in all treatment sites of all control animals, acute and chronic, with all chronic control animals requiring early termination or resulting in an early death. There were no perforations observed in any test animals that survived to scheduled sacrifice; one test animal expired early due to non-device related complications.
Conclusions: This study demonstrates that J-Plasma® can be safely utilized to treat small and large porcine intestine with no perforations observed. This device is an alternative tool in the surgical treatment of intra-abdominal cancer when removing cancer from peritoneal and bowel surfaces.
Introduction
Ovarian cancer accounts for 5% of cancer deaths among women, causing more deaths than any other gynecologic cancer. In the United States in 2018, there were an estimated 22,240 new diagnosed cases and 14,070 deaths [1]. One of the most significant factors associated with survival in patients with ovarian cancer is the completeness of their initial cytoreductive surgery. Many studies have demonstrated that removal of all macroscopic disease is associated with prolonged survival and should be the goal of primary cytoreductive surgery [2-6]. Removing visible disease from the bowel through bowel resection is associated with significant morbidity including longer hospital stays, higher surgical blood loss, higher rates of surgical complications, and higher costs [7]. A technology that allows for the safe and effective ablation of visible disease on the bowel without the need for bowel resection could improve patient outcomes by allowing for more complete disease removal during initial cytoreductive procedures without the morbidities associated with bowel resection.
Apyx Medical Corporation’s J-Plasma® helium-based plasma technology (J-Plasma) has FDA clearance for the cutting, coagulation, and ablation of soft tissue. The J-Plasma system consists of an electrosurgical generator unit (ESU), a handpiece, and a supply of helium gas. Radiofrequency (RF) energy is delivered to the handpiece by the ESU and used to energize an electrode. When helium gas is passed over the energized electrode, a helium plasma is generated which allows for conduction of the RF energy from the electrode to the patient in the form of a precise helium plasma beam. The energy delivered to the patient via the helium plasma beam is very precise and cooler in temperature in comparison to other surgical energy modalities such as the Argon Beam Coagulator (ABC) and standard RF monopolar energy. Previous studies comparing J-Plasma to the other energy sources such as ABC demonstrated less or comparable lateral and depth of thermal spread for J-Plasma in porcine peritoneum, bladder, and small intestine [8].
J-Plasma has been shown to be safe and effective in numerous surgical procedures where precise delivery of energy coupled with minimal thermal spread to adjacent tissue is important, such as ablation of endometriotic cells on fallopian tubes and ovaries (unpublished company data) and lysis of adhesions next to bowel or other vital structures. The demonstrated safety and efficacy of J-Plasma and its improved effects on tissue when compared to ABC make it a good candidate for use in cytoreductive procedures. A porcine model was chosen for this study, because pigs have been established through numerous studies in literature as a surrogate for human abdominal anatomy due to similarities in size, physiology, and response to surgical therapies.
Objectives
This was a ten (10) day survival study designed to compare the histologic effects of the argon beam coagulator (ABC, Conmed Corp., Largo, FL, USA) to J-Plasma (Apyx Medical, Clearwater, FL, USA) on porcine bowel.
Methods
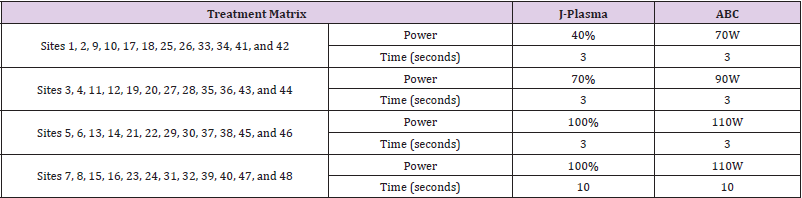
Seven (7) animals were randomly assigned a number 01-07 and underwent creation of a pneumoperitoneum and laparotomy. Animal procedures were reviewed and approved by the American Preclinical Society (APS) Insitutional Animal Care and Use Committed (IACUC), and the study was perfomed according to institutional guidelines established by APS. Segments of the jejunum, ileum, ascending colon, transverse colon, descending colon, and sigmoid colon of Animals 01-04 (one acute and three chronic) were treated with J-Plasma. Eight (8) J-Plasma treatment sites were created on each of the six (6) intestinal segments in the acute animal (48 treatment sites total) and six (6) J-Plasma treatment sites were created on each of the six intestinal segments in both chronic animals (36 treatment sites total in each animal). Each treatment site was demarcated by placement of interrupted non-absorbable 4-0 or 5-0 suture and spaced at least 4cm from an adjacent treatment site within the intestinal segment. The settings and duration of treatment are detailed in Table 1. Following treatment, Animal 01 was sacrificed approximately four hours after procedure completion for tissue analysis of the acute effect. Animals 02-03 were sacrificed 14 days following surgery. The identical procedure was then repeated in Animals 05-07 (one acute and two chronic) with ABC energy.
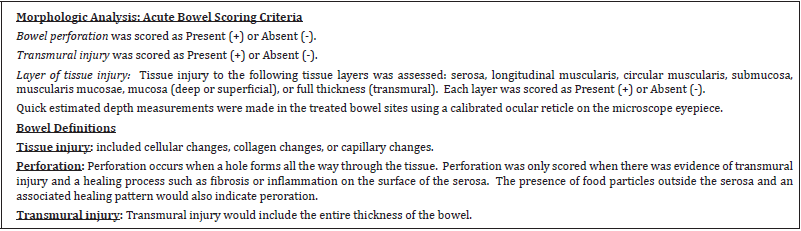
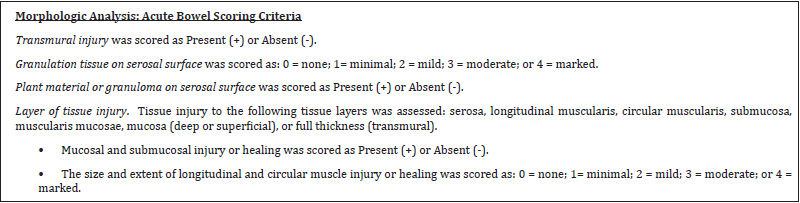
Histology was performed on each treatment site for depth of thermal injury, measured using morphometry, and injury to transmural layers: serosa, longitudinal muscularis, circular muscularis, submucosa, mucosa, or full thickness perforation. The Study Pathologist was blinded as to the treatment identification information (with the exception of the length of survival posttreatment) for each site during histopathologic slide review. After initial evaluation, treatment identification was unblinded in order to interpret data. The acute bowel scoring criteria and acute bowel data are located in Table 2. The chronic bowel scoring criteria and chronic bowel data are located in Table 3.
Results
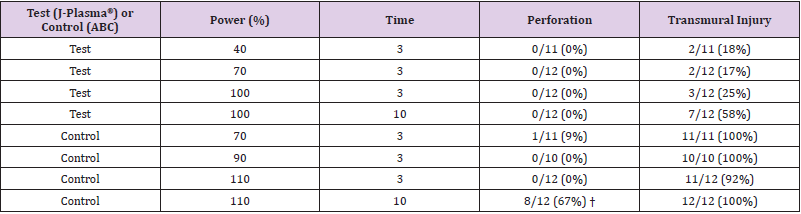
One J-Plasma (test) animal in the chronic arm of the study died on day one from non-device related complications; no data is included from this animal. The results presented are, therefore, from the three animals which survived. One of these animals was assigned to the acute arm of the study and two assigned to the chronic arm. For the acute animal, at a power setting of 40-100% and treatment times of 3 and 10 seconds, J-Plasma did not result in bowel perforation in any of the 47 treatment sites. Pathology evaluation showed transmural injury in 2/12 (17%) treatment sites at a power setting of 70% and 7/12 (58%) treatment sites at a power setting of 100% (Table 4). By comparison, at power settings of 70 -110% (conventional settings for ABC), bowel perforations were observed in 1/11 (9%) treatment sites at a power setting of 70% and in 8/12 (67%) treatment sites at a power setting of 110% (Table 4).
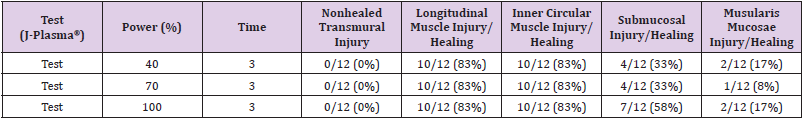
None of the animals treated with ABC in the chronic arm survived to 14 days; therefore, the only data available and presented for the chronic arm is that of animals treated with J-Plasma. Pathology evaluation details of the treatment sites in the chronic animal that survived to Day 14 is presented in Table 5. Of the 36 assessable treatment sites, there was no non-healed transmural site. Healing of longitudinal muscle injury was observed in 30/36 (83%) of the sites combined that were treated with 40-100% power settings. Healing of inner circular muscle injury was also observed in 83% of treatment sites, which is identical to the rate of healing observed in longitudinal muscle injury. Healing of submucosal injury was observed in 33%, 33%, and 58% of sites treated with 40%, 70%, and 100% power, respectively. Lastly, healing of muscularis mucosa injury was observed in 1/12 (8%) of sites treated at 70% power setting and in 17% of sites treated at 40 and 100% power settings.
Discussion
Metastatic intra-abdominal cancer commonly involves both small and large intestine. Therefore, debulking surgery for this cancer often involves intestinal surgery which ranges from resecting nodules or masses from the serosal surfaces of intestine to resecting bowel segments. Depending on the extent of dissection necessary, bowel surgery can be associated with considerable morbidity, yet the ultimate goal of the surgery is to achieve “no gross residual disease” because of its positive impact on survival 2,3. Morbidity can be reduced by limiting the extent of bowel dissection performed during debulking surgery. Medical devices such as the ABC and the cavitron ultrasonic surgical aspiratior (CUSA) have been utilized to remove tumor from peritoneal and bowel surfaces in an attempt to achieve optimal cytoreduction without resecting bowel. This study’s findings in a porcine model suggests that J-Plasma could be a safe addition to the array of devices commonly used during debulking surgery.
Porcine intestine is an acceptable surrogate for evaluating safety of any device intended for use in humans because of the similarities between porcine and human intestinal walls. One study assessed and compared the pinch force necessary for perforation in both human and porcine intestine [9]. The authors concluded that perforation forces between porcine and human small intestine were found to be similar and that it seems justified to perform experiments evaluating the safety of forceps on pig bowel tissue and extrapolate to human bowel. To this extent, the comparative safety profile demonstrated by J-Plasma in this study offers reassurance that the device could be safely used on human intestine during debulking surgery.
It is important to note that this experiment was carried out using the devices on smooth porcine intestinal wall that had no tumor deposits. In reality, the utility of the device at the time of surgery in humans will be strictly to remove tumor deposits not to treat the actual intestinal wall. This adds another layer of safety as the goal in the clinical setting would be to ablate/vaporize just the tumor. Like all devices utilizing thermal energy, adjacent spread of energy to neighboring tissues is unavoidable. What is more important is that such spread is not of sufficient intensity as to cause damage. In our study, we noted the impact of J-Plasma on the wall of the acute and chronic treatment sites, but no perforation resulted from such energy spread. Rather, pathology assessment of all the treatment sites in three chronic animals demonstrated progressive healing at all the treatment sites, another indicator of the safety of J-Plasma. In conclusion, our porcine intestinal model utilized in this study indicates that J-Plasma is safe to use on porcine intestine even at high energy without putting the animal in danger. J-Plasma should be considered another safe device that could be used to remove tumors from human intestine at debulking surgery.
Acknowledgements
I would like to thank Dr. Chi for his support and mentorship and Apyx Medical Corporation for their Sponsorship of this study.
References
- Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68: 7-30.
- Chang SJ, Bristow RE, Chi DS (2015) Role of aggressive surgical cytoreduction in advanced ovarian cancer. J Gynecol Oncol 26(4): 336-342.
- Chi DS, Eisenhauer EL, Lang J, Y Sonoda, D A Levine M, et al. (2006) What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol 103(2): 559-564.
- Eisenhauer EL, Abu Rustum NR, Sonoda Y, A Levine, Elizabeth A, et al. (2006) The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol Oncol 103(3): 1083-1090.
- Eisenkop SM, Friedman RL, Wang HJ (1998) Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecol Oncol 69(2): 103-108.
- Eisenkop SM, Spirtos NM (2001) Procedures required to accomplish complete cytoreduction of ovarian cancer: is there a correlation with "biological aggressiveness" and survival? Gynecol Oncol 82: 435-441.
- Salani R, Zahurak ML, Santillan A, Robert E Bristow (2007) Survival impact of multiple bowel resections in patients undergoing primary cytoreductive surgery for advanced ovarian cancer: a case-control study. Gynecol Oncol 107(3): 495-499.
- Pedroso J GM, Volker W (2014) J Plasma monopolar pencil, argon beam and CO2 laser electrosurgery: comparative evaluation of thermal spread in a porcine tissue model (White Paper). Apyx Medical Corporation.
- Heijnsdijk EA, van der Voort M, de Visser H (2003) Inter- and intraindividual variabilities of perforation forces of human and pig bowel tissue. Surg Endosc 17(12): 1923-1926.

 Research Article
Research Article