Abstract
Background and Purpose: The principle of thermally-induced contraction of collagen through denaturation and coagulation of soft tissue is well known in medicine and is used to achieve beneficial results in ophthalmology, orthopedics, varicose vein ablation, and cosmetic plastic surgery procedures. Once tissue is heated to the appropriate temperature, protein denaturation and collagen contraction occur resulting in a reduction of volume and surface area of the heated tissue. Recently, a helium-based plasma technology has been introduced for the percutaneous delivery of plasma energy for the purpose of soft tissue coagulation and contraction. The purpose of this research was to understand the internal and external tissue temperatures resulting from treatment with the helium plasma device and the impact of device settings on temperature during the coagulation and contraction of subcutaneous soft tissue.
Methods: Simulated use of the helium plasma device was performed on the abdomen of a live domestic cross pig following Klein solution infiltration and liposuction. Incisions through the epidermis and into the subcutaneous tissue plane were used as visualization windows to allow a FLIR camera to capture internal and external tissue temperatures simultaneously during activation. Internal and external tissue temperatures were recorded for various power and helium gas flow settings.
Results: For all but one treatment combination, the external tissue temperatures did not increase more than 3.6°C from baseline over the course of six treatment passes. Maximum internal tissue temperatures for 60% and 80% power settings exceeded 85°C for approximately 0.08 seconds while maximum temperatures for 40% power settings remained below 85°C.
Conclusions: Helium plasma power settings between 60% and 80% produce soft tissue coagulation and contraction by rapidly heating the treatment site to temperatures greater than 85°C for approximately 0.08 seconds. A power setting of 40% does not heat the tissue above 85°C and therefore is not adequate for soft tissue contraction at typical treatment speeds of 1cm/s used in a clinical setting. There were no distinguishable trends between the helium flow rate setting and internal and external tissue temperatures.
Keywords: Helium Plasma; Tissue Temperature;Apyx;Renuvion
Introduction
Energy in some form has been applied to tissue since the beginning of recorded history. The practice of applying heat to tissue with cauters was used for thousands of years as an invaluable method of controlling hemorrhage. Continuous improvement of methods for utilizing the beneficial effects of heat on tissue eventually led to the development of the basic concepts of electrosurgery we know today. In October of 1926, Dr. Harvey Cushing used an electrosurgical unit developed by Dr. William T. Bovie to successfully remove a highly vascularized brain tumor from a patient after previous failed attempts. Today, electrosurgical instruments are used in almost every surgical procedure performed worldwide [1]. Through this long history, the heat effects of the radiofrequency (RF) alternating current used in electrosurgery on cells and tissue have been well established. Normal body temperature is 37°C and, with normal illness, can increase to 40°C without permanent impact or damage to the cells of our body. However, when the temperature of cells in tissue reaches 50°C, cell death occurs in approximately 6 minutes [2]. When the temperature of cells in tissue reaches 60°C, cell death occurs instantaneously [3]. Between the temperatures of 60°C and just below 100°C, two simultaneous processes occur [1]. The first is protein denaturation leading to coagulation which will be discussed in more detail below. The second is desiccation or dehydration as the cells lose water through the thermally damaged cellular wall. As temperatures rise above 100°C, intracellular water turns to steam, and tissue cells begin to vaporize as a result of the massive intracellular expansion that occurs. Finally, at temperatures of 200°C or more, organic molecules are broken down into a process called carbonization. This leaves behind carbon molecules that give a black and/or brown appearance to the tissue [1].
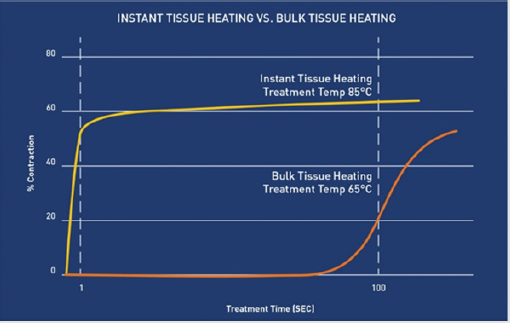
Understanding these heat effects of RF energy on cells and tissue can allow the predictable changes to be used to accomplish beneficial therapeutic results. Protein denaturation leading to soft tissue coagulation is one of the most versatile and widely utilized tissue effects. Protein denaturation is the process in which hydrothermal bonds (crosslinks) between protein molecules, such as collagen, are instantaneously broken and then quickly reformed as tissue cools [4]. This process leads to the formation of uniform clumps of protein typically called coagulum through a subsequent process known as coagulation [1]. In the process of coagulation, cellular proteins are altered but not destroyed and form protein bonds that create homogenous, gelatinous structures [1]. The resulting tissue effect of coagulation is extremely useful and most commonly used for occluding blood vessels and causing hemostasis [1]. In addition to causing hemostasis, coagulation results in predictable contraction of soft tissue [4-6]. Collagen is one of the main proteins found in human skin and connective tissue. The coagulation/denaturation temperature of collagen is conventionally stated to be 66.8°C, although this can vary for different tissue types [4]. Once denatured, collagen rapidly contracts as fibers shrink to one-third of their overall length [5]. However, the amount of contraction is dependent upon the temperature and the duration of the treatment. As depicted in Figure 1, the hotter the temperature the shorter amount of treatment time needed for maximal contraction [5]. For example, collagen heated at a temperature of 65°C must be heated for greater than 120 seconds for significant contraction to occur, but at a temperature of 85°C, maximal contraction occurs in approximately 0.044 seconds [5]. This principle of thermally-induced contraction of collagen through denaturation and coagulation of soft tissue is well known in medicine and is used to achieve beneficial results in ophthalmology [7], orthopedics [8], varicose vein ablation [9], and cosmetic plastic surgery procedures [10-20]. Once tissue is heated to the appropriate temperature, protein denaturation and collagen contraction occur resulting in a reduction of volume and surface area of the heated tissue [4-6].
Figure 1: Percent of collagen contraction as a function of time for different treatment temperatures [5].Hotter temperatures require less treatment time to achieve maximal contraction.
Background of Rf Technologies For Subcutaneous Clinical Applications: Recently, the use of thermal-induced collagen/tissue contraction has been expanded to minimally invasive procedures [17-22]. Laser-assisted lipolysis (LAL) and radiofrequencyassisted lipolysis (RFAL) devices have combined the removal of subcutaneous fat with soft tissue heating to address the tissue laxity that often results from fat volume removal [17-22]. These devices are placed in the same subcutaneous tissue plane as a standard suction-assisted lipolysis (SAL) cannula and are used to deliver thermal energy to coagulate the subcutaneous tissue including the fascia and the septal connective tissue [17-22]. The coagulation of the subcutaneous tissue results in collagen/tissue contraction (shrinkage) and changes in the mechanical behavior of the tissue [6]. The amount of contraction/shrinkage is well established in the scientific literature and has long been used as a method for quantifying the amount heat-induced damage to collagen and collagenous tissues [4].
More recently, a helium-based plasma technology (Renuvion®; Apyx™ Medical Corporation, Clearwater, FL) has been introduced for the percutaneous delivery of RF energy and helium plasma for cutting, coagulation, and ablation of soft tissue [23]. The system consists of an electrosurgical generator unit, a handpiece and a supply of helium gas. RF energy is delivered to the handpiece by the generator and used to energize an electrode. When helium gas is passed over the energized electrode, a helium plasma is generated which allows heat to be applied to tissue in two different and distinct ways [23]. First, heat is generated by the actual production of the plasma beam itself through the ionization and rapid neutralization of the helium atoms. Second, since plasmas are very good electrical conductors, a portion of the RF energy passes from the electrode to the patient and heats tissue by passing current through the resistance of the tissue, a process known as Joule heating [1,23].
The conductive helium plasma beam can be thought of as a flexible wire or electrode that “connects” to the tissue that represents the path of least resistance for the flow of the RF energy. Tissue that represents the path of least resistance is typically either the tissue that is in closest proximity to the tip of the device, or the tissue that has the lowest impedance (is the easiest to pass energy through). When used for the coagulation of subcutaneous soft tissue, this means that the energy from the helium plasma device is not directed or focused in any set direction when activated in the subdermal plane. If the path of least resistance is through the muscle fascia, the plasma energy will be directed to the muscle fascia. If the path of least resistance is through the fibroseptal network (FSN), the plasma energy will be directed there. As the tip of the device is drawn through the subdermal plane, new structures are introduced to the tip of the device and the path of least resistance is constantly changing [23]. As the energy is constantly finding a new preferred path, the plasma beam quickly alternates between treating the different tissue surrounding the tip of the device. This allows for 360° tissue treatment without the need for the user to direct the flow of energy. Since the FSN is typically the closest tissue to the tip of the device, most of the energy delivered results in coagulation and contraction of the fibroseptal bands. Published studies have shown that the majority of soft tissue contraction induced by subcutaneous energy delivery devices is due to its effect on the FSN [18,19]. Therefore, maximizing the energy flow to the FSN expedites the soft tissue contraction process.
Purpose: The purpose of this research utilizing a live porcine model is to understand the internal and external tissue temperatures resulting from treatment with the helium plasma device and the impact of device settings on temperature during the coagulation and contraction of subcutaneous soft tissue.
Material and Methods
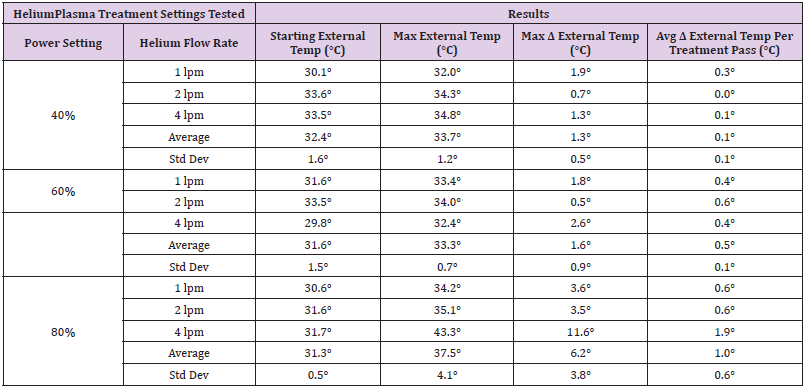
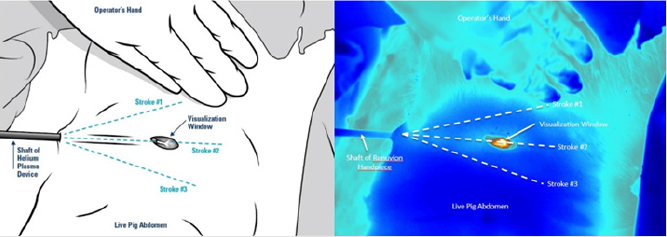
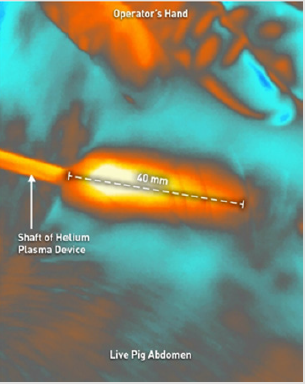
To simulate clinical conditions as closely as possible, Klein solution infiltration and liposuction was performed on the abdomen of a live female domestic cross pig. This animal experiment was conducted in compliance with the National Institutes of Health guide for the care and use of laboratory animals and was approved by the laboratory’s internal animal care and use committee prior to initiation. After completion of the liposuction, 1cm long incisions were made through the epidermis and dermis to allow for visualization of the subdermal tissue plane. These 1cm long incisions served as visualization windows that facilitated the use of a Forward Looking Infrared (FLIR) camera to simultaneously measure the internal and external tissue temperatures. Using a treatment speed of 1 cm/s, six treatment passes were performed using the helium plasma device at the various treatment settings listed in Table 1. For each of the treatment combinations tested, a single treatment pass consisted of three strokes of the device in the superficial subdermal plane. As detailed in (Figure 2), the first stroke was approximately 30° to the right of the visualization window, the second stroke passed directly underneath the visualization window, and the third stroke was approximately 30° to the left. During treatment, the FLIR camera was focused on the visualization window and the surrounding external tissue. Thermal imaging videos were captured and analyzed to extract maximum external tissue temperatures as well as internal tissue temperatures as a function of time for each treatment combination.
Figure 2: Image depicting the treatment method (2a left image) and a corresponding image captured from the FLIR thermal imaging videos (2b right image).The visualization window allows for simultaneous capture of the internal and external tissue temperatures.
Results
External Tissue Temperatures
The maximum temperatures recorded for the external tissue following six treatment passes are summarized in Table 1. Prior to treatment, the epidermal temperatures ranged from 29.8°C to 33.6°C. For all treatment combinations except for one, the external tissue temperature did not increase more than 3.6°C over the course of the six treatment passes. The treatment combination of 80% power, 4 liters per minutes (lpm) of helium flow saw an increase of 11.6°C over the baseline temperature resulting in a maximum external tissue temperature of 43.3°C. This apparent outlier in the data was analyzed further and is explained below in the Discussion section.
Internal Tissue Temperatures
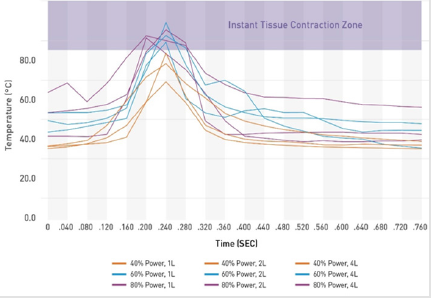
Since the internal temperatures rise and fall quickly as the energy is passed over the tissue, the maximum internal temperatures were evaluated as a function of time. Figure 3 shows typical curves for each treatment combination of the maximum internal temperature (°C) versus time (seconds). The heat cycle for a given treatment area consists of a rapid increase in temperature as the tip of the helium plasma device approaches, a maximum temperature as the tip passes directly over the area, and a rapid return to the baseline temperature as the tip moves away, all within a span of approximately 0.24 seconds. Maximum temperatures for the 60% and 80% power settings exceeded 85°C for approximately 0.08 seconds while maximum temperatures for the 40% power settings remained below 85°C.
Discussion
One of the main challenges associated with percutaneous delivery of energy for the purpose of thermal-induced collagen/ tissue contraction is the balance that must be achieved between heating the internal tissues enough to achieve the desired contraction while maintaining safe external tissue temperatures. Most commercially available RF devices work on the principle of bulk tissue heating [17-22]. In these devices, the energy is primarily directed into the dermis and the device is activated until a pre-set subdermal temperature in the range of 65°C is achieved and maintained across the entire volume of tissue, or the epidermal temperatures rise to unsafe levels. As shown above in Figure 1, at 65°C, the tissue being treated must be maintained at that temperature for a minimum of 120 seconds for maximal contraction to occur. Although these devices have proven effective in achieving soft tissue contraction [20], the process of heating the tissue to the treatment temperature and maintaining that temperature for extended periods can be time consuming [19]. In addition, during this process, the heat eventually conducts to the epidermis requiring constant monitoring of epidermal temperatures to ensure they do not exceed safe levels [17-22].
The results of this study demonstrate that the helium plasma device heats tissue differently than the bulk-heating RF devices. The helium plasma device heats each treatment location to temperatures greater than 85°C for approximately 0.08 seconds. As shown in Figure 1, 0.08 seconds is long enough to achieve maximal contraction. Unlike with bulk tissue heating, the tissue surrounding the treatment location remains at much cooler temperatures resulting in rapid cooling after the application of the energy through conductive heat transfer. In the process, less heat is transferred to the epidermis resulting in safe external temperature increases of 3.6°C or less over as many as six treatment passes.
The external tissue temperature increase of 3.6°C or less referenced above excluded one outlier in the data at the treatment settings of 80% and 4 lpm which saw an increase of 11.6°C. Further analysis of the thermal imaging video for this data point revealed that the highest temperatures were seen within 40mm from the incision site (Figure 4). Since all treatment strokes converge at the incision site, it is important to stop activation of the energy as the tip approaches the incision site to avoid over-heating in this area. For this particular data point (the last one taken during this study), not as much care was taken to stop activation in this area resulting in energy being delivered to the tissue with all three strokes performed with each of the six treatment passes (18 doses of energy total). The external tissue temperatures in all other areas for this treatment combination were equivalent to those seen for 80% power at 1 lpm and 2 lpm helium flow rates. Although this outlier in the data was caused by a mistake in the execution of the protocol, the current design of the helium plasma device now includes indication lines on the shaft within 40mm of the tip to provide notification to the user to stop activation as the lines begin to exit the incision during use (Figure 5).
Figure 4: FLIR video image showing highest temperature within 40mm from the incision site for the outlier in the external tissue data.
Figure 5: Tip distance indicators added as a safety feature to the current design of the helium plasma device.
Conclusion
This study demonstrates that helium plasma device settings between 60% and 80% power produce soft tissue coagulation and contraction by rapidly heating the treatment site to temperatures greater than 85°C for approximately 0.08 seconds. The tissue surrounding the treatment site remains at much cooler temperatures resulting in rapid cooling after the application of the energy through conductive heat transfer. Because of these unique heating and cooling properties, immediate contraction of the fibroseptal network can be achieved without unnecessarily heating the full thickness of the dermis and maintaining safe epidermal temperatures of less than 47°C. This translates into the clinical benefits of faster treatment times combined with greater soft tissue contraction when compared to devices that rely on the bulk heating process. The 40% power setting does not heat the tissue above 85°C. and therefore is not adequate for soft tissue contraction at typical treatment speeds of 1 cm/s used in a clinical setting.
There were no distinguishable trends between the helium flow rate setting and internal and external tissue temperatures. As a result, helium flow rates ranging from 1.5 lpm to 3.0 lpm should be selected to reduce the potential for residual subcutaneous helium or crepitus. Lower flow rates should be used in confined spaces of the anatomy.
References
- MG Munro (2012) Fundamentals of Electrosurgery Part I: Principles of Radiofrequency Energy for Surgery, in: LS Feldman, PR Fuchshuber, DB Jones (Eds.), The SAGES Manual on the Fundamental Use of Surgical Energy (FUSE). Springer New York, USA, pp:15-59.
- SN Goldberg, GS Gazelle, EF Halpern, WJ Rittman, PR Mueller, et al. (1996) Radiofrequency tissue ablation: Importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad Radiol 3(3): 212-218.
- S Thomsen (1991) Pathologic analysis of photothermal and photomechanical effects of laser-tissue interactions. Photochem Photobiol. 53(6): 825-835.
- NT Wright, JD Humphrey (2002) Denaturation of collagen during heating: An irreversible rate process. Annu Rev Biomed Eng. 4: 109-128.
- SS Chen, NT Wright, JD Humphrey (1997) Heat-induced changes in the mechanics of a collagenous tissue: isothermal free shrinkage. J Biomech 109(4): 372-378.
- SS Chen, JD Humphrey (1998) Heat-induced changes in the mechanics of a collagenous tissue: pseudoelastic behavior at 37° C. J Biomech 31: 211-216.
- MB McDonald (2005) Conductive Keratoplasty: A Radiofrequency-based Technique for the Correction of Hyperopia. Am J Ophthalmol 142(2): 512-536.
- SL Obrzut, P Hecht, K Hayahsi, GS Fanton, G.Thabit III, MD Markel, et al. (1998) The effect of radiofrequency on the length and temperature properties of the glenohumeral joint capsule. Arthroscopy 14(4): 395-400.
- TH Teruya, JL Ballard (2004) New approaches for the treatment of varicose veins. Surg Clin North Am, 84(5):1397-1417.
- EV Ross, JR McKinlay, RR Anderson (1999) Why does carbon dioxide resurfacing work? A review. Arch Dermatol 135(4): 444-454.
- ES Gardner, L Reinisch, GP Stricklin, DL Ellis DL (1996) In vitro changes in non-facial human skin following CO2 laser resurfacing: a comparison study. Lasers Surg Med 19(4): 379-387.
- SN Doshi, TS Alster (2005) Combination radiofrequency and diode laser for treatment of facial rhytides and skin laxity. Cosmet Laser Ther 7(1): 11-15.
- A Fatemi, MA Weiss, RA Weiss (2002) Short-term histologic effects of nonablative resurfacing: results with a dynamically cooled millisecond-domain 1320nm Nd:YAG laser, Dermatol Surg, 28(2) :172-176.
- FA Mayoral (2007) Skin tightening with a combined unipolar and bipolar radiofrequency device. J Drugs Dermatol 6: 212-215.
- TS Alster, SN Doshi, SB Hopping (2004) Combination surgical lifting with ablative laser skin resurfacing of facial skin: a retrospective analysis. Dermatol Surg 30(9): 1191-1195.
- B Zelickson, D Kist, E Bernstein, DB Brown, S Ksenzenko, et al. (2004) Histological and ultrastructural evaluation of the effects of a radiofrequency-based nonablative dermal remodeling device: a pilot study. Arch Dermatol 140: 204-209.
- T Hsu, M Kaminer (2003) The use of nonablative radiofrequency technology to tighten the lower face and neck. Semin Cutan Med Surg 22: 115-123.
- M Paul, G Blugerman, M Kreindel, RS Muholland (2011) Three-dimensional radiofrequency tissue tightening: a proposed mechanism and applications for body contouring. Aesthetic Plast Surg 35: 87-95.
- D Hurwitz, D Smith (2012) Treatment of overweight patients by radiofrequency-assisted liposuction (RFAL) for aesthetic reshaping and skin tightening. Aesthetic Plast Surg 36: 62-71.
- DI Duncan (2013) Nonexcisional Tissue Tightening: Creating Skin Surface Area Reduction During Abdominal Liposuction by Adding Radiofrequency Heating. Aesthetic Surgery Journal 33(8): 1154-1166.
- US FDA 510(k) Premarket Notification Database, 510(k) Clearance K171593.
- US FDA 510(k) Premarket Notification Database, 510(k) Clearance K173582.
- US FDA 510(k) Premarket Notification Database, 510(k) Clearance K191542.

 Research Article
Research Article