Abstract
Background/Aims: Transient gastrointestinal infections frequently precede functional bowel disorders with altered visceral sensory function. The aim of our study was a) to demonstrate the long-term effect of a chemically induced colitis on visceral sensory function in response to phasic colorectal distensions (CRD) and b) to analyze the impact of different opiate receptor agonists (ORA) and the NMDA antagonist ketamine on modulation of visceral hypersensitivity following chemical colitis in a rat model.
Methods: In forty male Lewis rats, 6 weeks after induction of trinitrobenzene sulfonic acid (TNB) colitis (colitis group) versus saline (control group), electromyographic recordings of CRD were performed. Different ORA and/or ketamine were administered intraperitoneally.
Results: The chemically induced colitis was followed by persistent visceral hypersensitivity. TNB-treated animals showed a significant increase of the visceromotor response (VMR) (p=0.006). While κ-ORA U50.488 had the strongest effect on VMR (p<0.05), the NMDA antagonist ketamine neither alone nor in combination led to a significant decrease of VMR to CRD.
Conclusions: In our visceral hypersensitivity model in rats and at the applied doses, the typical side effects of the μ-ORA fentanyl and morphine could be avoided by use of κ-ORA without any loss of analgesic effect on visceral nociception.
Keywords: Chemical Colitis; Functional Bowel Disorders; Opiates, Rat Model; Visceral Nociception
Introduction
Irritable bowel syndrome (IBS) is a gastrointestinal syndrome characterized by chronic abdominal pain and altered bowel habits in the absence of any organic cause. Although IBS represents 25 to 50 percent of all referrals to gastroenterologists [1] and is the second most frequent cause for work absenteeism after common cold [2]. It remains a chronic condition without any generally accepted and successful therapy for all IBS patients. Clinical studies have traditionally brought attention to colonic motility as potential cause for the symptoms associated with IBS. One study described 3cpm (contractions per minute) in the unstimulated colon in patients with IBS compared to 6cpm in controls [3]. Other studies have described selective hypersensitization of visceral afferent nerves in the gut in IBS patients based upon the observation that many patients with IBS have lower tolerance for rectal balloon distension than controls [4,5]. Interestingly, epidemiological studies have revealed in IBS patients an increased prevalence of persistent alterations in visceral nociception following acute gastroenteritis. The extent to which these observations might contribute to the pathogenesis of IBS in the general population remains unclear, as only a relatively limited number of patients have been studied.
Since IBS generally presents as a complex of symptoms, treatment is based on the predominant symptom and subtype (constipation vs. diarrhea vs. mixed vs. unclassified). Lifestyle and dietary modification are of cornerstone importance and considered first line treatment. Further, in patients with IBS and abdominal pain, antispasmodics and antidepressants, anxiolytics and antibiotics are used [6,7]. Limited evidence suggests that antienkephalinase agents, somatostatin analogues, alpha (2) receptor agonists, selective serotonin reuptake inhibitors, probiotics and herbal treatments may be useful in IBS patients [8]. However, some of the serotonin receptor agonists/antagonists used for causal therapy in IBS have been removed from the market, due to severe side effects such as ischemic colitis and serious cardiovascular complications. Hence at the time being, research is still in progress for a specific causal therapy with as little unwanted side effects as possible. Nociceptive mechanisms of potential interest in this context are opioidergic pathways, the glutamate receptor axis (e.g. NMDA receptor antagonists) as well as a combination of these two pathways. In the study of Burton and Gebhart the pain threshold to CRD in rats with or without acute inflammation of the colon was elevated after administration of the κ-opiate receptor agonist (ORA) U50.488 [9]. Others found that the analgesic effect of fentanyl can be potentiated by a co-application of ketamine [10].
Endogenous opioids are distributed throughout the whole organism and produce a diversity of biologic effects such as analgesia, motoric or stress responses. Opioids bind to three different receptor subtypes, the κ-, μ- and δ-opioid receptors (OR). κ-OR are mainly found in the tunica muscularis as well as in the myenteric plexus (Auerbach) where they have an impact on analgesia and motility. μ-OR of the gastrointestinal tract are primarily located in the tunica mucosa, submucosa, as well as in the submucosal plexus (Meissner). Finally, the δ-OR are found in the submucosal and myenteric plexus and the peripheral nerve fibers [11]. Given the hypomotile effect of opioids, their use in treatment of IBS may be accompanied by problematic unwanted effects such as constipation because of diminished intestinal passage and secretion [12,13]. Further gastrointestinal side effects may include emesis and nausea, but also reduced motility of the gallbladder and elevated tonus of the sphincter oddi. Thus, it would be desirable to identify an opioidergic substance with maximal analgesic potency and minimal side effects. This holds especially true because nausea, vomiting and constipation often represent symptoms of IBS patients. In our study, we focused on two main objectives. First, to reproduce the known long-term effect of a chemically induced colitis on visceral hypersensitivity to tonic distension in Lewis rats in an animal model with phasic colorectal distensions as stimulus in order to simulate the hyperalgesic status of IBS patients following acute gastrointestinal infection. Secondly, to study the impact of different opioid receptor agonists and the NMDA antagonist ketamine on long-term visceral hypersensitivity following induction of TNB colitis. We aimed to compare the clinically used μ-ORA’s fentanyl and morphine, the δ-ORA SNC80 as well as the NMDA antagonist ketamine with the κ-ORA U50.488.
Material and Methods
Animals
As laboratory animals male Lewis rats (Harlan, Horst, Netherlands) weighing between 200 and 320g are used. They housed individually in temperature-controlled rooms with a 12h dark–light cycle. Access to water and food was free. Before induction of the TNB colitis as well as before implantation of the electrodes, the animals were fasted for 24 hours. The state department of veterinary services approved the study.
Drugs
Every drug is given in a 1ml solution. When a combination of two drugs were used, each drug is given in a 0.5ml solution to reach the targeted volume of 1ml. The dose of each drug was chosen based upon the literature. For the κ-ORA 1S,2S-U50.488 (18mg/kg BW i.p. [14], Sigma-Aldrich Chemie, Buchs (SG), Switzerland) H2O dist. was used as solvent. The μ-ORA fentanyl (0.05mg/kg BW i.p. [15], central pharmacy of the university hospital of Berne, Switzerland) and morphine (3mg/kg BW i.p [15,16], central pharmacy of the university hospital of Berne, Switzerland) and the NMDA receptor antagonist ketamine (5mg/kg BW i.p. [17], central pharmacy of the university hospital of Berne, Switzerland) were dissolved in physiological saline solution (0.9%). For the δ-ORA SNC80 (10mg/ kg BW i.p. [18], Sigma-Aldrich Chemie, Buchs (SG), Switzerland) DMSO (Sigma-Aldrich Chemie, Buchs (SG), Switzerland) was used as solvent.
Induction of Colitis
To induce a local colorectal inflammation, 0,4ml of 100% 2,4,6-trinitro-benzene-sulfonic (TNB) acid (Sigma-Aldrich Chemie, Buchs (SG), Switzerland) and 0,4ml of 50% ethanol (test group) or an equal volume of 0,9% saline (control group) was injected via a nelathon catheter (outer diameter 3mm; Medicoplast, Illingen, Germany) under combined anaesthesia with ketamine hydrochloride (1ml/kg BW i.p., Ketamin 10%®, Sanofi-Ceva, Duesseldorf, Germany) and xylazin hydrochloride (0,05ml/animal i.p., Xylazin 2%®, Sanofi-Ceva, Duesseldorf, Germany). The catheter was inserted 8cm into the colon. This model has been frequently used and is well characterized [19].
Implantation of EMG Electrodes
To quantify the visceromotor response (VMR) to phasic colorectal distension (CRD), the activity of the abdominal wall musculature was recorded by electromyography (EMG). For this purpose, we implanted small-sized electrodes into the lateral abdominal wall of the animals following median laparotomy under combined anesthesia with ketamin hydro-chloride (1ml/kg BW i.p., Ketamin 10%®, Sanofi-Ceva, Duesseldorf, Germany) and xylazin hydrochloride (0,05ml/animal i.p., Xylazin 2%®, Sanofi-Ceva, Duesseldorf, Germany). As bipolar electrodes, teflon-insulated silver wires (Cooner Wire Co., Chatsworth, CA/USA) were used, passing through the abdominal wall musculature. After surgical intervention, animals could recover for one week. After this week, animals were put together in groups of five with food and water ad libidum.
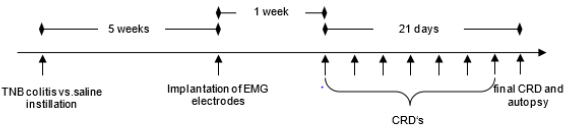
Timetable
First forty male Lewis rats were divided into two groups of 20 animals, a TNB colitis (test) and a saline group (control). Five weeks after induction of a TNB colitis or colorectal injection of saline, the EMG electrodes were implanted. After a recovery period of one-week, phasic CRD experiments were started. The VMR to CRD was studied with eight test substances or their combinations (saline, U50.488, fentanyl, morphine, SNC80, ketamine, ketamine/ fentanyl or ketamine/mor-phine). In the process of the testing, the animals were given a recovery period of three days after each series of stimulation (Figure 1). The order of the injected substances was identical in all animals and every substance was used in each animal. Therefore, the sequence of administration was not randomised.
Figure 1: Timetable. After rectal instillation of TNB or saline the animals were given a recovery period of 5 weeks till the implantation of the EMG electrodes. After another week, colorectal distensions which were always three days apart, were started.
Measurement of the Visceromotor Response
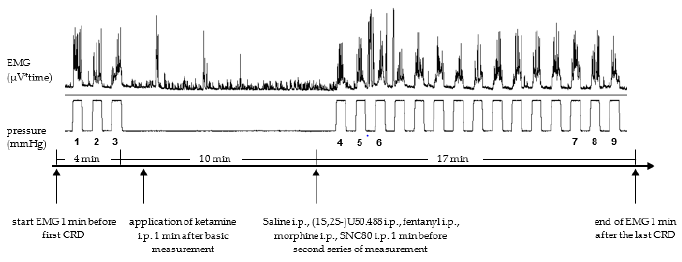
The CRDs were performed with pressure-controlled air inflation of a 4,5cm flexible balloon, which was connected to a computer-controlled barostat (Distender Series IITM barostat, G&J Electronics, Willowdale, Ontario/Canada). The VMR to the phasic CRDs was recorded and measured with an EMG device (ISOLAB/IDAA, Intestinal Data Acquisition and Analysis, Fa. Standard Instruments, Karlsruhe, Deutschland). The VMR to CRDs in the rat has been extensively characterized by Ness [20]. Under slight anesthesia with isoflurane, the lubricant-coated balloon (sonography lubricant, central pharmacy of the university hospital of Berne, Switzerland) was inserted into the colorectum, positioned about 1cm proximal of the anus and fixed to the tail with adhesive tape. Afterwards, the animals were placed in a Boreman cage and the EMG electrodes connected with the EMG recording system. The study protocol consisted of phasic CRDs (every minute 40mmHg for a period of 30sec). Before the application of a test substance, we performed three CRD’s as basic measurement (measurement A). The application of the test substance was followed by 15 CRD’s. The first three and the last three CRD’s were denoted measurement B and C, respectively. The first 3 CRDs and the subsequent generated 15 CRDs were 11 min apart. The EMG was recorded during the whole trial, starting 1 min before the first CRD and ending 1 min after the last CRD (Figure 2). The drugs were administered intraperitoneal, either 1 min (Ketamine) after measurement A or 1min before measurement B (saline, 1S,2S-U50.488, £Fentanyl, morphine and SNC80).
Figure 2: Schematic representation of the experimental protocol. The phasic CRD’s were repeated every minute (30 sec. duration with a distension pressure of 40mmHg). 1,2 and 3 are referred to as measurement A, 4 -6 as measurement B and 7-9 as measurement C.
Statistical Analyses
To evaluate the effect of a chemically induced colitis on visceral nociception, the difference in EMG activity (in μV) over time, resulting in an area under the curve (μV*time), between period C and B in the test group (TNB) versus the control group (saline) was compared. In this first part of the experimental protocol, a possible adaptation in the course of time was analysed. Therefore, only the first and the last three distensions were studied. To quantify the impact of the different ORA’s and ketamine, the differences in EMG activity between the measurement C and A were compared. Since ketamine is hypothesized to act only on activated pain pathways, the last three measurements of the repetitive distensions were evaluated. This protocol was consistently applied to all the substances to compare the results. Therefore, a negative value represents a decrease of VMR and thus a decrease in visceral sensory function. A mean value for the three consecutive distensions was calculated. Due to the non-normal distribution, the Wilcoxon rank sum test as well as the Wilcoxon signed rank test for statistical analysis were used. All values are given as the median of the area under the curve. A p-value of ≤ 0.05 was considered significant.
Results
Influence of TNB colitis on VMR
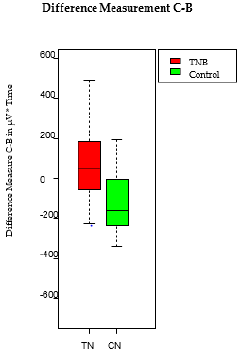
After i.p. administration of saline, an increase of the VMR to CRD was recorded during the following 15 repetitive CRD’s in the colitis group whereas the control group showed a decrease resulting in a significant difference between the test results (differences of the measurement C and B) of the treatment and the control group (49 vs. -161; p=0.006) (Figure 3).
Figure 3: Increased visceral sensory function during the 15 repetitive CRD in the TNB colitis animals. Caused by the chemically induced TNB colitis, visceral nociception is significantly higher compared to the control group (49 vs. -161; p = 0.006). This hypersensitivity translated into an increased VMR. The boxplot shows the median, the 25th and 75th percentile, as the minimum and the maximum. T=TNB colitis animals, C=control animals N=saline
Influence of different ORA’s on VMR in TNB Animals
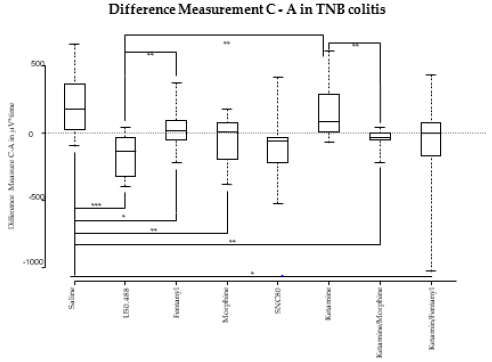
κ-ORA: Compared to saline-treated animals, VMR to CRD significantly decreased from measurement A to C after application of U50.488 in TNB animals (183 vs. -135; p=0.003).
μ-ORA: After application of both fentanyl and morphine (18 vs. 183; p=0.012 for fentanyl, 4 vs. 183; p=0.002 for morphine) a significant reduction of VMR in TNB-treated animals was recorded compared to saline-treated rats.
δ-ORA: Compared to saline, the application of the δ-ORA SNC80 did not have any statistically significant effect on the VMR (183 vs. -105; p 0.067) (Figure 4).
Figure 4: Differences of the measurements C versus A of the VMR in μV*time. Only the measurements of the TNB colitis animals are displayed. The boxplot shows the median, the 25th and 75th percentile, as the minimum and maximum. *= p-value 0.05, **= p-value 0.005, ***= p-value 0.0005
Influence of Ketamine on VMR in colitis Animals
The comparison of VMR to CRD during measurement A and C did not show any significant reduction after application of ketamine compared to saline (85 vs. 183; p=0.376) (Figure 4).
Influence of Ketamine Combined with μ-ORA on VMR in colitis Animals
The combination of ketamine with fentanyl caused a significantly greater decrease of the VMR (0 vs. 183; p=0.027) compared to saline. Similar findings were seen for the combination of ketamine with morphine compared to saline (-31 vs. 183; p=0.002). The comparison of the effect of ketamine combined with fentanyl to the κ-ORA U50.488 shows a slightly stronger effect of U50.488 (0 vs. -135; p=0.094). The same counts for the combination of ketamine with morphine compared to U50.488 (-31 vs. -135; p=0.068) (Figure 4).
Discussion
Since alterations in visceral sensory function are considered a major etiological factor in the pathogenesis of IBS, opioidergic neurotransmitter pathways represent an attractive target to treat IBS. Unfortunately, unwanted side effects of several opioids prevent their widespread use in this context. Given the fact that these side effects depend on the type of ORA, the effect of different ORA’s on visceral nociception deserves particular attention to identify a suitable ORA with maximal impact on visceral sensory function and minimal side effects. The focus of our present study was the characterization of different opiate subtypes for pain modulation following induction of chemical colitis in a rat model. Therefore, the comparison of TNB versus control animals served as confirmation of prior results of tonic CRD and proof of concept for the present set-up of phasic CRD as suitable animal model for IBS. Based upon these data, the purpose of this study was not to determine a specific and IBS-limited effect of opiate subtypes in IBS (in contrast to control animals). On the contrary, the study aimed at defining an opiate subtype with maximal analgesic effect and, at the same time, minimal gastro-intestinal side effects as possible therapeutic tool for the treatment of IBS. In our study with male Lewis rats, a chemically induced colitis with TNB caused persisting visceral hyper-sensitivity thus simulating visceral hypersensitivity seen in IBS patients following acute gastrointestinal infection. Both the κ-ORA U50.488 and the μ-ORA’s fentanyl and morphine reduced VMR to CRD with U50.488 displaying the most analgesic potential. No further analgesic effect could be observed by combining ORA’s with the NMDA antagonist ketamine.
Influence of TNB Colitis on VMR to CRD
In our series of phasic CRD tests, it was possible to reproduce the already known influence of TNB colitis on the VMR to tonic distensions. Contrary to our earlier experiments, in the present study we modified our experimental set-up and used phasic distensions as stimulus. In the control animals, we observed a reduction of the EMG response during the 15 phasic CRD’s after an i.p. application of saline. This finding can be explained by adaptation to the distension stimulus in healthy animals. This mechanism appears to fail in TNB-pretreated animals. In these latter animals, VMR to CRD raises significantly during the series of distensions. Similarly, to our results, among others, Zhou et al. described an increase in sensitivity and hyperalgesia following the induction of a TNB colitis in the rat model [21]. This was tested by using mechanic and thermal stimulation of the hind-paws. Zhou et al. concluded that this somatic hypersensitivity reflects tonic impulse input from the inflamed colon along with central sensitization.
Influence of Different ORA’s on VMR in Colitis Animals
κ-ORA: Both in TNB and control animals, the application of the κ-ORA U50.488 led to a significant reduction of the VMR to the CRD stimulus with a greater potency in the TNB-pretreated colon. The analgesic effect of κ-ORA on visceral nociception has been controversially discussed in the literature [22,23]. Danzebrink et al. postulated that only the systemic but not the intrathecal application of the κ-ORA led to an antinociceptive effect in visceral pain [24]. Other reports suggested that subcutaneous, but not central administration of κ-ORAs inhibited the VMR to CRDs [25]. In their model, Larsson et al. used mice and distensions were performed with increasing as well as repeated steady distensions lasting 10s and with 5 min intervals. U-69.593 was used as κ-ORA. Kitamura et al. showed that intravenous administration of U50.488 produced strong visceral antinociception in the un inflamed colon [26]. This effect was completely antagonized by naloxone. It has been shown that κ-, but not μ- and δ-ORAs, given intra-arterial attenuated the response to the noxious stimulus. For this purpose, Su et al. performed recordings of mechanosensitive pelvic nerve afferent fibres of the decentralized S1 dorsal root in anesthetized rats. This antinociceptive activity of κ-ORAs such as U50.488 during inflammation is partly mediated by NO [27]. The peripheral injection of U50.488H was enhanced by the injection of the NOreleasing agent FK409 and reversed by the administration of the NO scavenger PTIO [28]. Another group demonstrated that the peripheral antinociception induced by the κ-ORA bremazocine was mediated by L-arginine/NO/cGMP activation [29]. The long-term persistence of the analgesic effect after induction of chemical colitis could be explained by the theory that the corresponding receptors are either not localized or restricted to the site of inflammation or they are not affected by the colitis. Other groups suggest a peripheral upregulation of κ-opioid receptors in or following colonic inflammation [30-32].
μ-ORA Fentanyl: Following an intraperitoneal application of fentanyl, we observed a decreased pain inhibitory effect on visceral nociception compared to morphine. We are not able to explain why fentanyl leads to a lesser reduction in pain than morphine in our setup. Theoretically, the use of both ORA at different concentrations could have changed this finding.
μ-ORA Morphine: In our study, the administration of morphine was associated with a significant pain reduction to CRD in TNBtreated animals. Galantine et al. discovered that the μ-opioid morphine is highly active as analgesic against peritoneovisceral pain states in rats [33]. Similar effects have been described earlier, where systemic injections of morphine dose-dependently inhibited the VMR to CRD in rats with uninflamed or inflamed colons [22].
δ-ORA SNC80: In our study, application of the δ-ORA SNC80 reduced the EMG response to the visceral stimulus. Although this effect did not reach statistical significance in our study due to the largely dispersed data, it supports earlier findings by Sengupta et al. who described in a behavioural study that the δ-ORA SNC80 attenuated the responses of pelvic nerve afferent fibers in an uninflamed as well as in an inflamed colon but with significantly greater potency in the inflamed colon [27]. The greater potency of κ-ORAs in the TNB-inflamed condition suggests a peripheral upregulation of κ-OR‘s in colonic inflammation. The existence of distinct subtypes of δ-opioid receptors, δ1 and δ2, has been proposed by several research groups [32,34]. This hypothesis has been supported by the introduction of specific agonists and antagonists for both receptor subtypes. Bilsky et al. [35] reported that antinociception in response to intracerebroventricular application of SNC80 was fully antagonized by the DALCE/Cys- DELT combination but only partially blocked when these δ1- and δ2- receptor antagonists were separately given [36]. Therefore, these authors postulated that SNC80 is an unspecific δ-agonist. Pacheco et al. described the peripheral occurrence of δ1- and δ2- receptors and their antinociceptive function as result of the activation of the L-arginine/NO/cGMP pathway [37].
Influence of Ketamine on VMR in Colitis Animals
In our study, the intraperitoneal application of ketamine as non-competitive antagonist of the NMDA receptor [38,39] did not lead to any statistically significant decrease of the VMR to CRD. One possible explanation could be that the VMR in our pain model was not mediated by NMDA receptors at all. It can also be argued that the applied pressure for CRD in our experimental setup did not translate into a pain response high enough to activate the glutamate pathways. This might indicate that the application of ketamine is only effective after the onset of pain. This excludes a prophylactic use of the NMDA antagonist. Alternatively, the missing effect of ketamine in areas of chronic inflammation might be the result of an up-regulation of spinal NMDA receptors. As described by Kolhekar [40], spinal NMDA receptors are directly involved in local mechanisms that lead to enhanced spinal transfer of visceral nociceptive information and thus may participate in mechanisms leading to central hyperexcitability and visceral hyperalgesia. Zhou et al. also suggest a role for colonic myenteric plexus NMDA receptors in the development of neuronal plasticity and visceral hypersensitivity in the colon [21]. Up-regulation of NMDA receptor subunits may reflect part of the basis for chronic visceral hypersensitivity in conditions such as post-infectious irritable bowel disease. Thus, in our study, the used dose of ketamine could have been below the necessary level to show a significant effect.
Influence of Ketamine Combined with μ-ORA on VMR in Colitis Animals
Nadeson et al. showed in their work the possibility to potentiate the impact of intrathecal fentanyl by additional administration of ketamine and to reduce the amount of fentanyl used by a coapplication of ketamine [10]. In our study, we were not able to increase the antinociceptive effect of fentanyl by adding ketamine when both drugs were given by a non-spinal way. This combination has also been studied by several other research groups with mainly contradictory results [41-43]. The reason for these confounding results remains unclear. In our experiments, we were not able to further reduce the VMR to CRD by co-application of ketamine. This discordance of findings might be a result of the different models. Hirota et al. postulated a competitive interaction of ketamine with the μ- and κ-OR as functional μ- and κ-OR-antagonist that causes a non-opioidergic analgesic mechanism [44].
Limitations
Variability of the data can at least partially be explained by the difference in behaviour of the animals even before the beginning of the measurements. Some were calm, while others were already agitated resulting in higher baseline electromyographic activity and EMG measurements. Some animals did not survive the entire protocol. Thus, the total number of animals in each test series differed between 11 and 15. Another potential weak point of the present study is the lack of dose-response curves for the different substances which were tested. The respective concentrations for each test substance were determined by literature search. The aim of a follow-up study must be to confirm the conclusions of our study by including dose-response curves. In this context, both the absolute and relative expression of opiate receptor subtypes in the colon should be quantified in colitis and control animals. Finally, all substances were given in the same order in every animal, therefore no randomisation was performed. Thus, an adaption of the test animals to the CRD stimulus can not be excluded.
The irritable bowel syndrome is a very common disease. However, even in the year 2019, it cannot be treated satisfyingly. Postinfectious hyperalgesia may play a crucial role in the development of IBS at least in a subpopulation of IBS patients. The pathways of how to counteract this altered post-infectious visceral nociception have not been fully characterised yet. In earlier studies, we were able to demonstrate in Lewis rats that a chemically induced inflammation of the colon mucosa causes long-lasting visceral hyperalgesia in response to tonic visceral stimuli [45-47]. In our present series of tests, we were able to confirm these findings in an experimental set-up with phasic distensions. We also demonstrated that, at the applied doses, the κ-opioid receptor agonist U50.488 has the most potent analgesic effect on viscero-sensory function to CRD in Lewis rats following induction of a chemical colitis compared to the μ-ORA fentanyl and morphine, the δ-ORA SNC80 and the NMDA antagonist ketamine. Furthermore, we could show that the μ-ORA morphine also possesses a positive effect on the visceral hyperalgesia in the TNB-colitis rats. In our study, the application of ketamine alone or a combination of ketamine and the ORA’s missed to reduce or further reduce the VMR compared to the application of opioids alone. To conclude, our experiments demonstrate a strong analgesic effect of the selective κ-ORA U50.488 on long-term nociception in response to colorectal stimulation in a chemically induced colitis model. This is particularly noteworthy given the fact that this opiate receptor agonist unfolds its analgesic effect without the unwanted side effects of other opiate subtypes.
Acknowledgements
The statistical analysis was performed in close collaboration with the Institute for Mathematical Statistics of the University of Berne, Switzerland. This Study was supported by a grant-in-aid of the University of Berne and by unrestricted educational grants of the Novartis Stiftung für medizinisch-biologische Forschung, AstraZeneca, Nycomed and Solvay (all grants of JMG).
Conflicts of Interest
None declared.
Source of Funding
This Study was supported by a grant-in-aid of the University of Berne and by unrestricted educational grants of the Novartis Stiftung für medizinisch-biologische Forschung, AstraZeneca, Nycomed and Solvay (all grants of JMG).
References
- Everhart JE, PF Renault (1991) Irritable bowel syndrome in office-based practice in the United States. Gastroenterology 100(4): 998-1005.
- Schuster MM (1991) Diagnostic evaluation of the irritable bowel syndrome. Gastroenterol Clin North Am 20(2): 269-278.
- Snape WJ, GM Carlson, S Cohen (1976) Colonic myoelectric activity in the irritable bowel syndrome. Gastroenterology 70(3): 326-330.
- Bouin M, Victor Plourde, Michel Boivin, Monique Riberdy, France Lupien, et al. (2002) Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology 122(7): 1771-1777.
- Whitehead WE (1990) Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology 98(5 Pt 1): 1187-1192.
- Munjal AB Dedania, BD Cash (2019) Current and emerging pharmacological approaches for treating diarrhea-predominant irritable bowel syndrome. Expert Opin Pharmacother 21(6): 1-9.
- Niewinna K, A Zielinska, J Fichna (2019) Recent advances in the pharmacological management of constipation predominant irritable bowel syndrome. Expert Opin Pharmacother 21(1): 73-84.
- Chang HY, EC Kelly, AJ Lembo (2006) Current gut-directed therapies for irritable bowel syndrome. Curr Treat Options Gastroenterol 9(4): 314-323.
- Burton MB, GF Gebhart (1998) Effects of kappa-opioid receptor agonists on responses to colorectal distension in rats with and without acute colonic inflammation. J Pharmacol Exp Ther 285(2): 707-715.
- Nadeson R (2002) Potentiation by ketamine of fentanyl antinociception. I. An experimental study in rats showing that ketamine administered by non-spinal routes targets spinal cord antinociceptive systems. Br J Anaesth 88(5): 685-691.
- Lembo A (2006) Peripheral opioids for functional GI disease: a reappraisal. Dig Dis 24(1-2): 91-98.
- Lembo AJ, Brian E Lacy, Marc J Zuckerman, Ron Schey, Leonard S Dove, et al. (2016) Eluxadoline for Irritable Bowel Syndrome with Diarrhea. N Engl J Med 374(3): 242-253.
- Cash BD (2017) Safety of Eluxadoline in Patients with Irritable Bowel Syndrome with Diarrhea. Am J Gastroenterol 112(2): 365-374.
- Stoffel EC, Catherine M Ulibarri, John E Folk, Kenner C Rice, Rebecca M Craft (2005) Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. J Pain 6(4): 261-274.
- Redwine KE, KA (2003) Trujillo, Effects of NMDA receptor antagonists on acute mu-opioid analgesia in the rat. Pharmacol Biochem Behav 76(2): 361-372.
- Holtman JR, X Jing, EP Wala (2003) Sex-related differences in the enhancement of morphine antinociception by NMDA receptor antagonists in rats. Pharmacol Biochem Behav 76(2): 285-293.
- Suarez-Roca H, Jose Antonio Silva, Jose Luis Arcaya, Luis Quintero, William Maixner, et al. (2006) Role of mu-opioid and NMDA receptors in the development and maintenance of repeated swim stress-induced thermal hyperalgesia. Behav Brain Res 167(2): 205-211.
- Gallantine EL, TF Meert (2005) A comparison of the antinociceptive and adverse effects of the mu-opioid agonist morphine and the delta-opioid agonist SNC80. Basic Clin Pharmacol Toxicol 97(1): 39-51.
- Hoffmann P, J Zeeh, J Lakshmanan, V Wu, F Procaccino, et al. (1997) Increased expression of transforming growth factor alpha precursors in acute experimental colitis in rats. Gut 41(2): 195-202.
- Ness TJ, GF Gebhart (1988) Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res 450(1-2): 153-169.
- Zhou Q, Donald D Price, Robert M Caudle, G Nicholas Verne (2008) Visceral and somatic hypersensitivity in a subset of rats following TNBS-induced colitis. Pain 134(1-2): 9-15.
- Sengupta JN (1999) Effects of kappa opioids in the inflamed rat colon. Pain 79(2-3): 175-185.
- Junien JL, P Riviere (1995) Review article: the hypersensitive gut--peripheral kappa agonists as a new pharmacological approach. Aliment Pharmacol Ther 9(2): 117-126.
- Danzebrink RM, SA Green, GF Gebhart (1995) Spinal mu and delta, but not kappa, opioid-receptor agonists attenuate responses to noxious colorectal distension in the rat. Pain 63(1): 39-47.
- Larsson M (2003) A model for chronic quantitative studies of colorectal sensitivity using balloon distension in conscious mice -- effects of opioid receptor agonists. Neurogastroenterol Motil 15(4): 371-381.
- Kitamura TM Ogawa, Y Yamada (2009) The individual and combined effects of U50,488, and flurbiprofen axetil on visceral pain in conscious rats. Anesth Analg 108(6): 1964-1966.
- Sengupta JN, X Su, GF Gebhart (1996) Kappa, but not mu or delta, opioids attenuate responses to distention of afferent fibers innervating the rat colon. Gastroenterology 111(4): 968-980.
- Nozaki-Taguchi N, T Yamamoto (1998) The interaction of FK409, a novel nitric oxide releaser, and peripherally administered morphine during experimental inflammation. Anesth Analg 86(2): 367-373.
- Amarante LH, ID Duarte (2002) The kappa-opioid agonist (+/-)-bremazocine elicits peripheral antinociception by activation of the L-arginine/nitric oxide/cyclic GMP pathway. Eur J Pharmacol 454(1): 19-23.
- Hughes PA, Joel Castro, Andrea M Harrington, Nicole Isaacs, Melissa Moretta, et al. (2014) Increased kappa-opioid receptor expression and function during chronic visceral hypersensitivity. Gut 63(7): 1199-1200.
- Grundy D (2004) Peripheral opiate action on afferent fibres supplying the rat intestine. Neurogastroenterol Motil 16(Suppl 2): 29-37.
- Vaughn LK (1990) Differentiation between rat brain and mouse vas deferens delta opioid receptors. Eur J Pharmacol 177(1-2): 99-101.
- Gallantine EL, TF Meert (1991) Antinociceptive and adverse effects of mu- and kappa-opioid receptor agonists: a comparison of morphine and U50488-H. Basic Clin Pharmacol Toxicol 103(5): 419-427.
- Negri L (1991) Evidence for two subtypes of delta opioid receptors in rat brain. Eur J Pharmacol 196(3): 335-336.
- Portoghese PS (1992) Delta opioid antagonist activity and binding studies of regioisomeric isothiocyanate derivatives of naltrindole: evidence for delta receptor subtypes. J Med Chem 35(22): 4086-4091.
- Bilsky EJ, SN Calderon, T Wang, RN Bernstein, P Davis, et al. (1995) SNC 80, a selective, nonpeptidic and systemically active opioid delta agonist. J Pharmacol Exp Ther 273(1): 359-366.
- Pacheco DF, Gláucia ML Reis, Janetti N Francischi, Maria SA Castro, Andrea C Perez, et al. (2005) Delta-Opioid receptor agonist SNC80 elicits peripheral antinociception via delta (1) and delta (2) receptors and activation of the l-arginine/nitric oxide/cyclic GMP pathway. Life Sci 78(1): 54-60.
- Hustveit, OA Maurset, I Oye (1995) Interaction of the chiral forms of ketamine with opioid, phencyclidine, sigma and muscarinic receptors. Pharmacol Toxicol 77(6): 355-359.
- Mody I, JF MacDonald (1995) NMDA receptor-dependent excitotoxicity: the role of intracellular Ca2+ release. Trends Pharmacol Sci 16(10): 356-359.
- Kolhekar R, GF Gebhart (1996) Modulation of spinal visceral nociceptive transmission by NMDA receptor activation in the rat. J Neurophysiol 75(6): 2344-2353.
- Carstensen M, AM Moller (2010) Adding ketamine to morphine for intravenous patient-controlled analgesia for acute postoperative pain: a qualitative review of randomized trials. Br J Anaesth 104(4): 401-406.
- Malec D, M Mandryk, S Fidecka (2008) Interaction of memantine and ketamine in morphine- and pentazocine-induced antinociception in mice. Pharmacol Rep 60(2): 149-155.
- Sarton E (2001) The involvement of the mu-opioid receptor in ketamine-induced respiratory depression and antinociception. Anesth Analg 93(6): 1495-1500.
- Hirota K (1999) Stereoselective interaction of ketamine with recombinant mu, kappa, and delta opioid receptors expressed in Chinese hamster ovary cells. Anesthesiology 90(1): 174-182.
- Adam B, Tobias Liebregts, Juergen M Gschossmann, Constanze Krippner, Franziska Scholl, et al. (2006) Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain 123(1-2): 179-186.
- Gschossmann JM (2002) Effect of transient chemically induced colitis on the visceromotor response to mechanical colorectal distension. Eur J Gastroenterol Hepatol 14(10): 1067-1072.
- Gschossmann JM, T Liebregts, L Buenger, M Ruwe, G Gerken, G Holtmann, et al. (2004) Long-term effects of transient chemically induced colitis on the visceromotor response to mechanical colorectal distension. Dig Dis Sci 49(1): 96-101.

 Research Article
Research Article