Abstract
Nanotechnology has become one of the most talented technologies useful in all areas of science. Metal nanoparticles produced by nanotechnology have received global attention due to their widespread claims in the biomedical and physiochemical fields. Recently, synthesizing metal nanoparticles using plants has been extensively studied and has been recognized as a green and efficient way for further exploiting microorganisms as convenient manufactories. The current study designed to investigate the ability of Bauhinia variegate aqueous extract in the bio reduction of silver salt during the nanoparticle synthesis and evaluation of UV–visible spectroscopy as well as Transmission Electron Microscopy (TEM) of the synthetized nanoparticles.
Keywords: Silver Nanoparticles; Bauhinia Variegate; Transmission Electron Microscope
Introduction
Silver nanoparticles (Nps) have showed to be most effective because of its good antimicrobial efficacy against bacteria, viruses and other eukaryotic micro-organisms [1]. Silver is one of the basic elements that make up our planet. It is a rare, but naturally occurring element, marginally harder than gold and very ductile and flexible. Pure silver has the uppermost electrical and thermal conductivity of all metals and has the lowermost contact resistance. Silver can be present in four different oxidation states: Ag0, Ag2+, Ag3+. The former two are the most plentiful ones, the latter are unstable in the aquatic environment [2,3]. Metallic silver itself is insoluble in water, but metallic salts such as AgNO3 and Silver chloride are soluble in water [4]. Metallic silver is used for the surgical prosthesis and splints, fungicides and coinage. Soluble silver compounds such as silver slats, have been used in treating mental illness, epilepsy, nicotine addition, gastroenteritis and infectious diseases including syphilis and gonorrhea [5].
Although acute toxicity of silver in the environment is dependent on the availability of free silver ions, investigations have shown that these concentrations of Ag+ ions are too low to lead toxicity [6]. Metallic silver appears to pose minimal risk to health, whereas soluble silver compounds are more readily absorbed and have the potential to produce adverse effects [7]. The wide variety of uses of silver allows exposure through various routes of entry into the body. Ingestion is the primary route for entry for silver compounds and colloidal silver proteins. Dietary intake of silver is estimated at 70-90μg/day. Since silver in any form is not thought to be toxic to the immune, cardiovascular, nervous or reproductive system, it is not considered to be carcinogenic [8], therefore silver is relatively non-toxic [9].
Several methods have been used for the preparation of silver nanoparticles which can be physical, chemical, or biological methods. Earlier methods used for the synthesis of silver nanoparticles were toxic and hazardous chemicals were used for their synthesis. Thus, the use of eco-friendly processes, for the synthesis of silver nanoparticles is known as ‘‘Green synthesis’’. Green synthesis is preferred over conventional synthesis because it is ecofriendly, costeffective, single-step method that can be easily scaled up for large scale synthesis and does not require high pressure, temperature, energy, and toxic chemicals [10]. Many researchers have reported the use of materials such as plant leaf extract, root, stem, bark, leaf, fruit, bud, and latex [11] for the synthesis of silver nanoparticles. In producing nanoparticles using plant extracts, the extract is simply mixed with a solution of the metal salt at room temperature. The reaction is complete within minutes. Nanoparticles of silver, gold and many other metals have been produced this way [12]. The nature of the plant extract, its concentration, the concentration of the metal salt, die pH, temperature and contact time are known to affect the rate of production of the nanoparticles, their quantity, and other characteristics [13].
Morphologies and sizes of AgNPs depend greatly on the concentration of the solution. For instance, their distribution and size vary with the concentration of both the polysaccharides and the precursor metal salts. It is well established that the properties of AgNPs are greatly dependent on their morphology. The morphological change is a consequence of complex combinations of molecular, surface, and crystalline characters [14]. Considering electromagnetic radiation of AgNPs; several studies have shown that AgNPs absorb electromagnetic radiation in the visible region from 380 to 450 nm by means of a phenomenon known as the excitation of localized surface plasmon resonance (LSPR) [15]. (LSPR) is an optical phenomenon generated by a light wave trapped within conductive nanoparticles (NPs) smaller than the wavelength of light. The phenomenon is a result of the interactions between the incident light and surface electrons in a conduction band [16].
Materials and Methods
Collection of Plant Materia
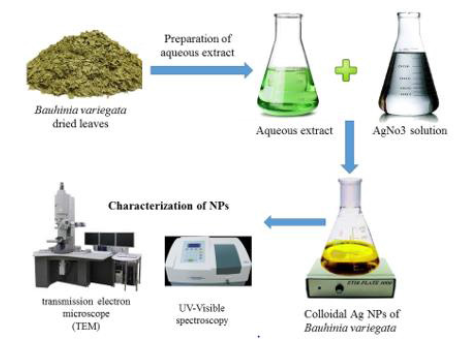
Bauhinia variegata (Figure 1) fresh leaves were collected from the garden of Botany department, faculty of science, Suez Canal University and authenticate botanist from faculty of science, Suez Canal University, Ismailia, Egypt.
Biosynthesis of Nps From Bauhinia Variegate Aqueous Extract
The broth solution of fresh leaves was prepared by taking 10 g of thoroughly washed and finely cut leaves in a 300mL Erlenmeyer flask along with 100mL of sterilized double-distilled water and then boiling the mixture for 5 min before finally decanting it. The extract was filtered with Whatman filter paper no. 1 and stored at −15°C; it could be used within 1 week. The filtrate was treated with aqueous 1 mM AgNO3 (21.2mg of AgNO3 powder in 125mL Milli-Q water) solution in an Erlenmeyer flask and incubated at room temperature. Eighty-eight milliliters of an aqueous solution of 1 mM silver nitrate was reduced using 12 mL of leaf extract at room temperature for 10 min, resulting in a brown-yellow solution indicating the formation of AgNPs [17].
UV–Visible Spectroscopy
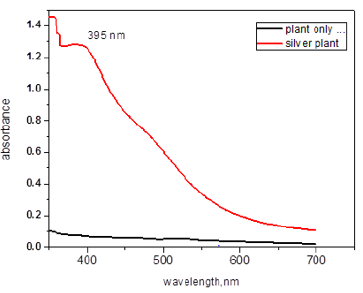
To examine the optical properties of nanoparticles UV-Vis spectroscopy is used aacording to the method described by Abbas [18]. In Figure 4, Bauhinia variegate synthetized nanoparticles (BVLSNPs) spectrum is observed in the figure characteristic absorption peak of the sample is noted that is 395nm and band gap is 3.26 eV. The supporting point is that there is no other peak is observed in whole a spectrum that means BVL-SNPs has successfully formed.
Transmission Electron Microscopy (TEM)
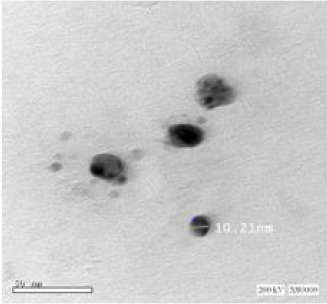
TEM analysis was performed on the analytical electron microscope (Jeol JEM 2100F) with a Field Emission Gun (FEG) operating at 200 kV. The samples were prepared by a simple colloid dropping on amorphous carbon film supported on cupper (Cu) grids. The electron dose used during all process was kept between 150 and 250 e/A2. s [19].
Results and Discussion
Qualitative analysis for the formation of silver nanoparticles can easily be followed using spectrophotometer. Color change was noted by visual observation in the BV leaf extracts when incubated with AgNO3 solution. the colorless solution of silver nitrate after the addition of boiled leaf extract of Bauhinia varigata change its color from colorless to light yellow and further yellow to yellowish brown (Figure 3). The excitation of Surface Plasmon Resonance (SPR) of silver nanoparticles exhibits yellowish-brown color indicating the formation of silver nanoparticles in the aqueous solution. The absorption spectrum of synthesized silver nanoparticles from Bauhinia varigata leaf extract at different wavelengths ranging from 300 to 700nm revealed a peak at 395 nm (Figures 4&5) shows a typical TEM image of the biosynthesized Bauhinia variegate nanoparticles produced. The TEM images of silver nanoparticles clearly show regularity in shape, which was spherical in nature and the average diameter of silver nanoparticles observed was 14 nm, but with variation in diameter, which ranged between 7.68-18.50nm. Formation of dispersed BVL-SNPS of uniform size can be observed from the size distribution histogram (Figure 5), an average particle size of 2.1 ± 0.2 nm could be estimated. These variations in shape and size of nanoparticles synthesized by biological systems are common [20]. Nanoparticles with smaller size can have promising applications in biomedicine due to the advantage of increased surface area [21]. Silver nanoparticles are being increasingly utilized in blood-contacting biomedical applications and devices. As with any new technology, thorough preclinical evaluation is necessary to ensure patient safety [22].
Figure 2: Schematic presentation for biosynthesis of silver nanoparticles using Bauhinia variegata aqueous extract.
Figure 3: Photograph showing the change in color during green synthesis. (A) Boiled B. variegata leaf extract. (BVLE). (B) Silver nitrate solution (AgNO3). (c) Synthesized silver nanoparticles from B. variegata leaf extract (BVL-SNPs).
Figure 4: UV–Visible absorption spectra of synthesized AgNPs from B. variegata aqueous leaf extract.
Biogenic synthesis is useful not only because of its reduced environmental impact [15] compared with some of the physicochemical production methods as well as it results in production of large quantities of nanoparticles that are free of contamination and have a well-defined size and morphology [22]. The ability of plant extracts to reduce metal ions has been known since the early 1900s, although the nature of the reducing agents involved was not well understood. In view of its simplicity, the use of live plants or whole plant extract and plant tissue for reducing metal salts to nanoparticles has attracted considerable attention within the last 30-years [23, 24]. Typically, a plant extract-mediated bio reduction involves mixing the aqueous extract with an aqueous solution of the relevant metal salt. The reaction occurs at room temperature and is generally complete within a few minutes. In view of the number of different chemicals involved, the bio reduction process is relatively complex. Nanoparticles are already used in numerous applications [25] including in vitro diagnostics, but their use in medicine is mostly on an experimental basis. Drugs bound to nanoparticles have been claimed to have advantages compared with the conventional forms of the drugs [26]. The size of the drug nanoparticle and its surface characteristics can be modified to achieve the desired delivery characteristics [20].
In synthesis of silver nanoparticles using a Bauhinia variegate leaf extract, the particles formed rapidly, and a stable size of 20-30nm was achieved. The nanoparticles are quite polydispersity and a layer of the organic material surrounding the synthesized Ag nanoparticles could explain the good dispersion of these nanoparticles in solution. Generally, the Ag nanoparticles synthesized by means of aqueous extracts are dispersed finely though some of them were renowned to be agglomerated. Particularly, the mainstream of the particles in the TEM images are not in physical contact with each other but appeared separated by the organic layer. Therefore, TEM images evidently indicate the coating of Ag nanoparticles with an organic layer. The presence of several polyphenolic components including flavonoids and terpenoids facilitated the reduction of Ag ions and also stabilized the surface of the resultant Ag nanoparticles [27].
Conclusion
The use of plant extracts is cheap, simply scalable, and environmentally harmless to yield metallic nanoparticles. It is predominantly suitable to make nanoparticles applicable as therapeutic agents. The synthesis of a plant extract can offer controlled sized and morphological nanoparticles. Nanoparticles in medicine, for example, are used in bandages as antimicrobial agents. Targeted drug delivery and clinical diagnosis technologies are being developed.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27(1): 76-83.
- Keat CL, Aziz A, Eid AM, Elmarzugi NA (2015) Biosynthesis of nanoparticles and silver nanoparticles. Bioresour Bioprocess 2(1): 1-11.
- Ramya M, Subapriya MS (2012) Green synthesis of silver nanoparticles. Int J Pharm Med Biol Sci 1(1): 54-61.
- Warrington PD (1996) Ambient water quality criteria for silver. Water Quality Branch.
- Galatage ST, Hebalkar AS, Gote RV, Mali OR, Killedar SG (2020) Silver Nano Particles by Green Synthesis: An Overview. Res J Pharm Technol 13(3): 1503-1510.
- Ratte HT (1999) Bioaccumulation and toxicity of silver compounds: a review. Environ Toxicol Chem An Int J 18(1): 89-108.
- Drake PL, Hazelwood KJ (2005) Exposure-related health effects of silver and silver compounds: a review. Ann Occup Hyg 49(7): 575-585.
- Panigrahi T (2013) Synthesis and characterization of silver nanoparticles using leaf extract of Azadirachta indica.
- Kleps I, Danila M, Angelescu A, Miu M, Simion M, et al. (2007) Gold and silver/Si nanocomposite layers. Mater Sci Eng C 27(5-8): 1439-1443.
- Zhang XF, Liu ZG, Shen W, Gurunathan S (2016) Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17(9): 1534.
- Pandey G, Madhuri S (2009) Some medicinal plants as natural anticancer agents. Pharmacogn Rev 3(6): 259.
- Katti AKS, Sharon M (2019) European Nano Knowledge That Led to Faraday’s Understanding of Gold Nanoparticles. Hist Nanotechnol From Pre-Historic to Mod Times pp. 141-212.
- Tejashri G, Amrita B, Darshana J (2013) Cyclodextrin based nanosponges for pharmaceutical use: A review. Acta Pharm 63(3): 335-358.
- Zhu Z, Su M, Ma L, Liu D, Wang Z (2013) Preparation of graphene oxide silver nanoparticle nanohybrids with highly antibacterial capability. Talanta 117: 449-455.
- Lee SH, Jun BH (2019) Silver nanoparticles: synthesis and application for nanomedicine. Int J Mol Sci 20(4): 865.
- Petryayeva E, Krull UJ (2011) Localized surface plasmon resonance: Nanostructures, bioassays and biosensing A review. Anal Chim Acta 706(1): 8-24.
- Gopinath V, MubarakAli D, Priyadarshini S, Priyadharsshini NM, Thajuddin N, et al. (2012) Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach. Colloids Surfaces B Biointerfaces 96: 69-74.
- Abbas Q (2019) Molecular Nanotechnology Understanding the UV-Vis Spectroscopy for Nanoparticles. J Nanomater Mol Nanotechnol 8(3): 8-10.
- Smith DJ (2015) Characterization of nanomaterials using transmission electron microscopy pp. 1-29.
- Albanese A, Tang PS, Chan WCW (2012) The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng 14: 1-16.
- Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, et al. (2014) “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae 6(1): 1.
- Majewski P, Thierry B (2007) Functionalized magnetite nanoparticles-synthesis, properties, and bio-applications. Crit Rev Solid State Mater Sci 32(3-4): 203-215.
- Mallikarjuna K, Narasimha G, Dillip GR, Praveen B, Shreedhar B, et al. (2011) Green synthesis of silver nanoparticles using Ocimum leaf extract and their characterization. Dig J Nanomater Biostructures 6(1): 181-186.
- Li G, He D, Qian Y, Guan B, Gao S, et al. (2012) Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int J Mol Sci 13(1): 466-476.
- Shukla AK, Iravani S (2018) Green synthesis, characterization and applications of nanoparticles. Elsevier.
- Mohanraj VJ, Chen Y (2006) Nanoparticles-a review. Trop J Pharm Res 5(1): 561-573.
- Marslin G, Siram K, Maqbool Q, Selvakesavan RK, Kruszka D, et al. (2018) Secondary metabolites in the green synthesis of metal Nanoparticles. Materials (Basel) 11(6): 940.

 Short Communication
Short Communication