Abstract
Objectives: The correlation between cancer-related thrombocytosis and worse survival has been described with a variety of solid neoplasms. However, only limited data are available on the prognostic significance of thrombocytosis in patients with head and neck tumors.
We aimed to investigate the association between the survival of patients with head and neck cancer and the elevated platelet count.
Methods: We conducted an analysis of the data from 339 patients with head and neck squamous cell carcinoma of various stages and locations. Preoperative platelet counts were analysed; thrombocytosis was defined as 300 G/L or higher. The influence of platelet count on survival was calculated with the Kaplan–Meier method, as well as with multivariate Cox regression.
Results: In patients with excessive thrombocytosis, survival was significantly worse even after adjusting the multivariate analysis for gender, age, as well as tumor stage, grade, and location. The magnitude of thrombocytosis differed among tumors of different anatomical location.
Conclusion: Thrombocytosis may be related to a worse survival in head and neck squamous cell carcinoma patients. The impact of elevated platelet count appears to vary with the anatomical location of the tumor – this feature may be worth further investigation.
Keywords: Head and Neck Neoplasms; Survival; Prognosis; Thrombocytosis
Abbreviations: HNSCC: Head and Neck Squamous Cell Carcinoma; CBC: Complete Blood Count; DFS: Disease-Free Survival; GITR: Glucocorticoid-Induced Tumor Necrosis Factor-Receptor Related Protein IL- 6: Interleukin-6; HPV: Human Papilloma Virus; MHC-I: Major Histocompatibility Complex Class I; NK: Natural Killer (cell); OS: Overall Survival
Introduction
Each year, 500,000 patients are diagnosed with head and neck tumors worldwide [1]. This condition, which is the 8th most common cause of death, contributes 6% of all malignancies [2]. Head and Neck Squamous Cell Carcinomas (HNSCC) require individualized therapy with three components-that is, surgery, irradiation, and chemotherapy. A prognostic marker for predicting therapeutic response or survival would be of great help in developing an appropriate treatment strategy. Possible candidates include conventional haematological indices such as Complete Blood Count (CBC) for example [3]. Haemoglobin levels have been found predictive of the primary response to irradiation, of the chance of local recurrence, as well as of overall (OS) [4], and of Disease-Free Survival (DFS) [5]. Recently, an association between elevated platelet count and earlier metastasis has been observed in a variety of solid neoplasms, including colorectal [6-10], gastric [11,12], cervical [13], ovarian [14-16], pancreatic [17,18], lung [19- 21], and breast [22] cancers. The exact pathomechanism behind the relationship between thrombocytosis and neoplasia is yet unknown to date. The most popular hypothesis postulates the presence of a paraneoplastic pathway in the background. The authors of this hypothesis found elevated serum Interleukin-6 (IL-6) levels in ovarian cancer. By enhancing the release of thrombopoietin from the liver, IL-6 enhances megakaryocytosis and this leads to thrombocytosis [23]. We studied the prognostic significance of platelet counts determined before initiating treatment in patients with oral, pharyngeal, or laryngeal malignancies in order to ascertain whether thrombocytosis is associated with a worse prognosis also in these tumors.
Materials and Methods
The study conforms with The Code of Ethics of the World Medical Association (Declaration of Helsinki), printed in the British Medical Journal (18 July 1964). The study was approved by the Scientific and Research Ethics Committee of the Hungarian Medical Research Council (Nr. 8951-3/2015/EKU (0444/15). We conducted a retrospective analysis on the clinical data of patients managed for primary malignancies of the oral cavity, the pharynx, and the larynx at two ear-nose-throat and oncology centres in Hungary (Uzsoki Street Hospital, and Medical Centre, Hungarian Defence Forces), between 2000 and 2014. The criteria for inclusion comprised the diagnosis of Squamous Cell Carcinoma (SCC) confirmed by histology, and the availability of laboratory findings obtained at least one month before treatment. The exclusion criteria were as follows: residual tumor or distant metastasis after surgery, the presence of another synchronous tumor, inflammatory conditions (pharyngeal-cutaneous fistula, pneumonia, wound infection, abscess, cholecystitis, cannule sepsis, endocarditis, urinary tract infection, Crohn’s disease, ulcerative colitis, etc.), thromboembolic events (deep vein thrombosis, pulmonary embolism, myocardial infarction), and concomitant therapy with corticosteroids. We excluded patients with leukocytosis or reduced CBC. Taking platelet aggregation inhibitors was not allowed for at least a month before treatment. Based on these criteria, we had to exclude 237 of 549 patients. They were staged according to the 7th edition of the TNM classification of the American Joint Committee on Cancer. The data available on the factors predisposing to malignancy (smoking, alcohol consumption, infection by human papilloma virus or the Epstein–Barr virus, etc.) were incomplete and hence, these were disregarded in our study. OS was defined as the period from the start date of treatment to the date of death from any cause – or to the last date of observation, whereas DFS was calculated as the time from the start date of treatment and to the date of the detection of recurrence.
When describing the characteristics of the study population, we calculated means and Standard Deviation (SD) for normally distributed data, and medians with Interquartile Range (IQR) for data with non-normal distribution. We applied cross-tabulation and non-parametric tests (Wilcoxon rank-sum test, Kruskall-Wallis test) when comparing the characteristics of subgroups within the sample. We analysed survival data using the Kaplan-Meier method, and compared the survival estimates of the subgroups with high or low platelet counts using the log-rank test. We defined 300 G/L as the cut-off value for high platelet count. When determining the optimal cutoff value, we followed the following process: we run logistic regression models with 3-year survival status as the dependent variable, and cut-off-values from the entire range of platelet-count rounded to 10. We calculated the area under ROC curve for each cut-off-value and selected the optimal cut-off based on the largest area under ROC. With this method, we selected 300 G/l as the optimal cut-off-value, a level somewhat lower than the clinical threshold of 400 G/l for thrombocytosis. We analysed 5-year overall survival and disease-free survival using multivariable Cox-proportional hazard-regression models, treating platelets as continuous, as well as discrete categorical variables. We tested the effect of multiple-category covariates using the Wald-test. We tested the proportional hazard assumption for the Cox-regression models according to the method proposed by Grambsch and Terneau [24] and tested goodness of fit on 4 quantiles of risk as proposed by May and Hosmer [4]. All statistical analyses were performed with the Stata 14.2 statistical software package [25].
Results
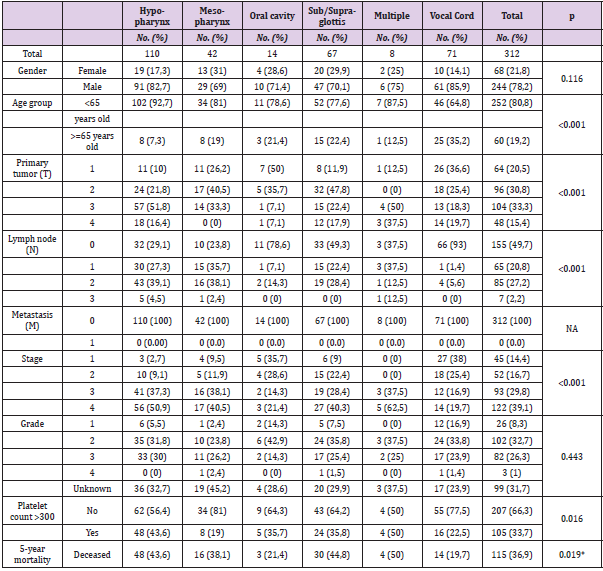
Platelet-count and overall survival data were available for 312, whereas information for calculating disease-free survival was known for 299 patients (95.8%). Mean age (SD) was 57 years (8.6); 21.8% of the patients (N=68) were female. Median followup time was 42.9 months (range 4.9-161.0), and 134 patients died during follow-up (42.9%). The cause of death was not related to the tumor in 31 patients (23.3%). 132 (42.3%) patients had diseaserecurrence. Median survival was 88.6 months (95% CI: 71.8-124.2), and median disease-free survival was 67.6 months (95% CI: 37.8- 87.0). The tumor locations were as follows hypopharynx (35.3%), mesopharynx (13.5%), oral cavity (4.5%), sub/supraglottis (21.5%), and vocal cords (22.8%). The location was multiple in 2.6% of subjects. Patient characteristics by tumor location are shown in Table 1. We found significant differences between various locations in terms of age, gender, primary tumor and lymph node status, the occurrence of thrombocytosis, and 5-year survival rate. The primary therapy was surgery in 226 patients (72.4%), irradiation and chemotherapy in 46 patients (14.7%), and radiotherapy in 40 patients (12.8%). Altogether 266 (85.3%), 125 (40.1%) and 111 (35.6%) patients underwent surgery, chemotherapy, and radiotherapy at any time during the follow-up, respectively.
Table 1: Patient characteristics by tumor location.
Note: *log-rank test chi2 (df=1) = 13.47, p=0.0193.
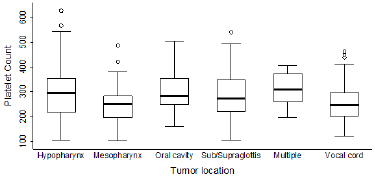
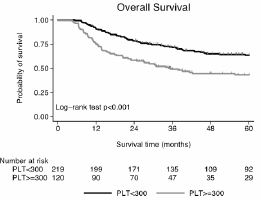
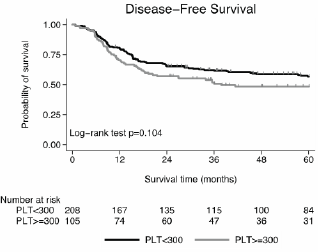
Platelet counts showed a right-skewed distribution (skewness=0.652, p<0.001). Median platelet-count was 264 G/L, the inter-quartile range was 116 (25-75th percentiles: 206.5- 322.5). Platelet count differed by the status, stage, and location of the primary tumor and the lymph-node spread (Kruskal-Wallis test p-values: p=0.015, p<0.001, p=0.031, p=0.025 respectively). More advanced primary tumor status, stage and lymph node spread were associated with higher median platelet-counts. The median platelet-count was 286 in hypopharyngeal, 251 in mesopharyngeal, 284 in oral, 273 in sub/supraglottic, 308 in multiple-site, and 245 in vocal cord tumors (Figures 1 & 2). Platelet counts over 300 G/L occurred in 43.6%, 19.1%, 35.7%, 35.8%, 50.0%, 22.5% of patients with tumors in these locations, respectively (Figure 1) and in 33.6% of the entire sample. The median survival of patients with platelet counts <300 G/L or >=300G/L was 98.7 months (95%CI: 80.7-124.4), and 35.4 months (95%CI: 30.9-), respectively. The difference was significant (Log-rank test chi2(df=1) =7.20, p=0.007). The Kaplan-Meier curves for 5-year overall survival are shown in Figure 2. Median disease-free survival was 78.5 (46.8-94.4) and 35.5 (17.5-) months for patients with platelet counts <300 G/l or >=300G/l, respectively. The difference was not significant (Log-rank test chi2(df=1)=1.70, p=0.192). The Kaplan- Meier curves for 5-year disease-free-survival are shown in Figure 3.
Figure 1: Box-plots of platelet count by tumor location.
Note: Thick lines in box-plots: medians, box borders: interquartile ranges, whiskers: the upper and lower fences, dots: outliers.
Figure 2: The relationship between platelet count and 5-year overall survival (Kaplan-Meier estimate).
Note: Vertical ticks on survival curves indicate censored cases.
Figure 3: The relationship between platelet count and 5-year disease-free survival (Kaplan-Meier estimate).
Note: Vertical ticks on survival curves indicate censored cases.
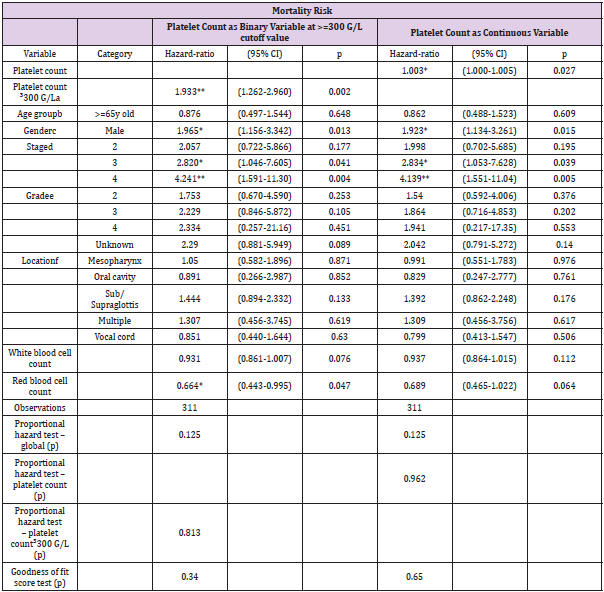
Platelet-count was a significant predictor of overall survival in the 5-year, multivariable Cox-proportional hazards regression analysis, after adjustment for the patients’ age, gender (Table 2), tumor stage and grade, as well as white and red blood cell counts. The Hazard Ratio (HR) was 1.003 (95%CI: 1.000-1.005, p=0.027). Among the control variables, male gender and advanced tumor status (stage 3-4) were associated with a significant increase of mortality risk. The effect of tumor stage on overall mortality was significant (Wald test p=0.011). The overall model was highly significant (LR test chi(17)2=49.40, p<0.0001). The proportional hazard assumption was not violated, and the model’s goodness of fit was acceptable. Using 300-G/L platelet-count as the cut-off-value, the results were similar to those of the analyses using platelets as a continuous variable. High platelet count was associated with a nearly two-fold increase in 5-year mortality risk. Male gender and advanced tumor stage were associated with an increased, while higher red blood cell count was associated with a reduced 5-year mortality risk. 5-year survival was not affected by tumor location in the multivariable analyses. Tumor recurrence was not affected significantly by platelet levels over 300 G/L (data not shown). Multivariable Cox regression showed no association between disease-free survival and platelet-count included as a continuous variable in the model. The proportional hazard assumption was not violated, while the significant goodness of fit test suggested poor model fit for both the binary and continuous Cox-regression analyses of disease-free survival.
Table 2: The results of the multivariable analysis of patient data (Cox regression analysis).
Note: Exponentiated coefficients
abase: Platelet count < 300G/L; bbase <65 y old; cbase: Female; dbase: Stage 1; ebase: Grade 1; fLocation: Hypopharynx
*p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
The relationship between thrombocytosis and a worse survival has been observed in several types of solid tumors [26,27]. The underlying pathomechanism is still unknown yet, but several hypotheses have been proposed, based on the following observations. On one hand, platelets can enhance tumor growth and stimulate angiogenesis by secreting proangiogenic cytokines [28]. On the other hand, platelets may be involved in the formation of metastases by cloaking tumor cells and thereby protecting them both from mechanical damage [29,30], and from the immune defences of the body [31,32]. By expressing immunoregulatory proteins on their surface, the platelets attached to the tumor cells can defend the latter against Natural Killer (NK) cells [33,34]. Moreover, platelets also express Major Histocompatibility Complex Class I (MHC-I) in abundant quantities. That is, adherent platelets confer a false phenotype to the tumor cells, and thereby interfere with the recognition of the malignant cells by the immune system [33]. This process is based on a paraneoplastic pathway, the starting point of which is the elevation of serum IL-6 level [23]. Because the pathomechanism has not yet been fully clarified, the possibility of reactive thrombocytosis cannot be excluded either. In order to eliminate this option, we excluded patients with leukocytosis or reduced CBC from our study. We confirmed the known correlation of reduced CBC and survival in patients with HNSCC. In general, a certain degree of anaemia develops before treatment in 30 to 50 per cent of cancer patients, and this proportion may increase to 50-70% during anti-tumor therapy. Anaemia impairs the patients’ quality of life on one hand, and reduces therapeutic efficacy on the other – thus, it may lessen the chances for cure and survival [35,36].
However, it is still controversial whether anaemia might be the underlying cause of thrombocytosis and poor survival in cancer patients. According to CHEN et al. [37], anaemia, monocytosis, and thrombocytosis were independent risk factors in their study population of patients with head and neck tumors – our study also confirmed this finding. Using 300-G/L platelet-count as the cut-offvalue, higher red blood cell count was associated with a reduced 5-year mortality risk. Besides this, multivariable Cox-proportional hazards regression analysis, after adjustment for the patients’ red blood cell counts, showed that platelet-count was a significant predictor of 5-year overall survival. Our study population comprised cancer patients undergoing therapy according to international standards for malignancies of diverse stages, grades, and locations. The analysis of our findings obtained in HNSCC patients with Kaplan–Meier’s log-rank test showed a significantly worse survival, if the platelet count exceeded 300 G/L. The multivariate analysis also detected – in contrast with results from other studies – a significant correlation between thrombocytosis and 5-year survival [38]. The previous studies investigating the relationship of thrombocytosis and HNSCC yielded results similar to ours. However, in a proportion of these studies, the number of eligible patients was reduced by limitations on the anatomical location of the tumor or on the use of a specific therapeutic modality, and the majority of these trials applied univariate analysis without performing a multivariate analysis in addition. An even bigger problem may be that the cut-off value for establishing thrombocytosis varied between 250 and 400 G/L [39-45] – this makes it impossible to compare the results and to draw an overall conclusion [46]. Remarkably, RACHIDI et al. [41], as well as CHEN et al. [37] found a correlation between both lower and higher platelet counts and poor survival. The head and neck region is regarded as an integrated system (consisting of the nasal cavity, the paranasal sinuses, the oral cavity, the salivary glands, the pharnyx and the larynx) and the predominant malignancy in this region is squamous cell carcinoma (HNSCC).
Notwithstanding this, the behaviour of tumor-associated thrombocytosis appears to be different in specific parts of this organ system. According to some observations, platelet counts are lower in patients with tumors of the oral cavity, or of the larynx (sub/supraglottic or vocal cord lesions) than in those with meso- or hypopharyngeal tumors. In the latter (hypopharyngeal) location, the correlation between platelet count and diseasespecific survival has been found to be particularly strong [38]. By contrast, other authors reported worse survival in patients with oral [39], or nasopharyngeal tumors [40]. Our study also found different platelet counts and 5-year survival rates depending on the anatomical location of the tumor. However, the multivariate Cox regression analysis did not confirm a significant relationship between anatomical location and survival. In view of the small number of cases, we did not perform a location-specific analysis of the relationship between thrombocytosis and survival. Male gender was associated with an increased 5-year mortality, though the association was not significant. LIN et al found similar difference in prognosis between males and females. High platelet count was associated with decreased OS in males but not in females. They performed gene expression analyses as well, which showed that females have higher immune cell infiltration in the tumor microenvironment [47]. Other studies have suggested greater activity in male platelets, which may lead to worse cancer prognosis [48-50]. In summary of our findings, we can conclude that – in a patient cohort with tumors with a variety of possible anatomical locations, stages, and treatment modalities – we found significant correlation between platelet count and worse survival in patients with head and neck tumors. However, the variable, anatomical location-dependent influence of platelet count may be a possible clue worth for further investigation, as this might bring us closer to understanding the relationship of thrombocytosis, solid tumors, and poor survival.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. (2012) Cancer incidence and mortality worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010.
- Wang L, Ye Er Dao Lai Ti Y, Yang G, Du J (2018) Effects of Regional and General Anesthesia on Survival in Head and Neck Cancer. Int J Pharmacol 14(4): 528-533.
- Hoff CM, Hansen HS, Overgaard M, Grau C, Johansen J, et al. (2011) The importance of haemoglobin level and effect of transfusion in HNSCC patients treated with radiotherapy--results from the randomized DAHANCA 5 study. Radiother Oncol 98(1): 28-33.
- Hosmer D, Lemeshow S, May S (2008) Applied Survival Analysis. John Wiley & Sons, Hoboken, USA.
- Prosnitz RG, Yao B, Farrell CL, Clough R, Brizel DM (2005) Pretreatment anemia is correlated with the reduced effectiveness of radiation and concurrent chemotherapy in advanced head and neck cancer. Int J Radiat Oncol Biol Phys 61(4): 1087-1095.
- Gu D, Szallasi A (2017) Thrombocytosis Portends Adverse Prognosis in Colorectal Cancer: A Meta-Analysis of 5,619 Patients in 16 Individual Studies. Anticancer Res 37(9): 4717-4726.
- Wang Y-H, Deng S-J, Yang Y-D, Yao N, Zhao J-M, et al. (2017) The pretreatment thrombocytosis may predict prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Biomark Med 11(2): 195-210.
- Baranyai Z, Jósa V, Tóth A, Szilasi Z, Tihanyi B, et al. (2016) Paraneoplastic thrombocytosis in gastrointestinal cancer. Platelets 27(4): 269-275.
- Josa V, Krzystanek M, Eklund AC, Salamon F, Zarand A, et al. (2015) Relationship of postoperative thrombocytosis and survival of patients with colorectal cancer. Int J Surg 18:1-6.
- Jósa V, Krzystanek M, Vass T, Lang T, Juhász V, et al. (2015) Thrombocytosis of Liver Metastasis from Colorectal Cancer as Predictive Factor. Pathol Oncol Res 21(4): 991-997.
- Hu C, Chen R, Chen W, Pang W, Xue X, et al. (2014) Thrombocytosis is a significant indictor of hypercoagulability, prognosis and recurrence in gastric cancer. Exp Ther Med 8(1): 125-132.
- Li F-X, Wei L-J, Zhang H, Li S-X, Liu J-T (2014) Significance of thrombocytosis in clinicopathologic characteristics and prognosis of gastric cancer. Asian Pac J Cancer Prev 15(16): 6511-6517.
- Cheng J, Zeng Z, Ye Q, Zhang Y, Yan R, et al. (2017) The association of pretreatment thrombocytosis with prognosis and clinicopathological significance in cervical cancer: A systematic review and meta-analysis. Oncotarget 8(15): 24327-24336.
- Nakao S, Matsumoto K, Komiya H, Tenjimbayashi Y, Sakurai M, et al. (2016) Paraneoplastic thrombocytosis as a prognostic marker in ovarian cancer. Paper presented at the international journal of gynecological cancer.
- Cozzi GD, Samuel JM, Fromal JT, Keene S, Crispens MA, et al. (2016) Thresholds and timing of pre-operative thrombocytosis and ovarian cancer survival: analysis of laboratory measures from electronic medical records. BMC Cancer 16: 612.
- Barber EL, Boggess JF, Van Le L, Kim KH, Bae-Jump VL, et al. (2015) Preoperative thrombocytosis and leukocytosis among ovarian cancer patients are associated with postoperative death. Gynecol Oncol 137: 43.
- Chadha AS, Kocak-Uzel E, Das P, Minsky BD, Delclos ME, et al. (2015) Paraneoplastic thrombocytosis independently predicts poor prognosis in patients with locally advanced pancreatic cancer. Acta Oncol 54(7): 971-978.
- Kocak E, Das P, Delclos M, Minsky BD, Crane C, et al. (2014) Paraneoplastic Thrombocytosis Is a Significant Independent Prognostic Factor for Overall and Progression Free Survival in Locally Advanced Pancreatic Cancer. Int J Radiat Oncol Biol Phys 90(1): S365.
- Kim M, Chang H, Yang HC, Kim YJ, Lee C-T, et al. (2014) Preoperative thrombocytosis is a significant unfavorable prognostic factor for patients with resectable non-small cell lung cancer. World J Surg Oncol 12: 37.
- Wang J, Luo M, Wu H, Sheng L, Su D, et al. (2013) Preoperative thrombocytosis as a predictor of unfavorable survival in resectable non-small cell lung cancer. J Clin Orthod 31(15_suppl): e18531-e18531.
- Maráz A, Furák J, Varga Z, Kahán Z, Tiszlavicz L, et al. (2013) Thrombocytosis has a negative prognostic value in lung cancer. Anticancer Res 33(4): 1725-1729.
- Rajkumar A, Szallasi A (2013) Paraneoplastic thrombocytosis in breast cancer. Anticancer Res 33(10): 4545-4546.
- Stone RL, Nick AM, Mc Neish IA, Balkwill F, Han HD, et al. (2012) Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med 366(7): 610-618.
- Grambsch PM, Therneau TM (1994) Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81(3): 515-526.
- Stata Corp (2015) Stata Statistical Software: Release 14. Stata Corp LP, College Station, TX.
- Lin RJ, Afshar-Kharghan V, Schafer AI (2014) Paraneoplastic thrombocytosis: The secrets of tumor self-promotion. Blood 124(2): 184-187.
- Zhang X, Lv Z, Yu H, Zhu J (2017) The clinicopathological and prognostic role of thrombocytosis in patients with cancer: A meta-analysis. Oncol Lett 13(6): 5002-5008.
- Buergy D, Wenz F, Groden C, Brockmann MA (2012) Tumor--platelet interaction in solid tumors. International journal of cancer 130(12): 2747-2760.
- Sierko E, Wojtukiewicz MZ (2007) Inhibition of platelet function: Does it offer a chance of better cancer progression control? Semin Thromb Hemost 33(7): 712-721.
- Gay LJ, Felding-Habermann B (2011) Contribution of platelets to tumour metastasis. Nat Rev Cancer 11(2): 123-134.
- Nieswandt B, Hafner M, Echtenacher B, Mannel DN (1999) Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59(6): 1295-1300.
- Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, et al. (2005) Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 105(1): 178-185.
- Placke T, Kopp HG, Salih HR (2011) Modulation of natural killer cell anti-tumor reactivity by platelets. J Innate Immun 3(4): 374-382.
- Erpenbeck L, Schon MP (2010) Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood 115(17): 3427-3436.
- Ludwig H, Van Belle S, Barrett-Lee P, Birgegard G, Bokemeyer C, et al. (2004) The European Cancer Anaemia Survey (ECAS): A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 40(15): 2293-2306.
- Birgegard G, Aapro MS, Bokemeyer C, Dicato M, Drings P, et al. (2005) Cancer-related anemia: Pathogenesis, prevalence and treatment. Oncology 68 Suppl 1: 3-11.
- Chen YP, Chen C, Mai ZY, Gao J, Shen LJ, et al. (2015) Pretreatment platelet count as a predictor for survival and distant metastasis in nasopharyngeal carcinoma patients. Oncol Lett 9(3): 1458-1466.
- Pardo L, Valero C, Lopez M, Garcia J, Camacho M, et al. (2017) The prognostic value of pretreatment platelet count in patients with head and neck squamous cell carcinoma. Auris Nasus Larynx 44(3): 313-318.
- Lu CC, Chang KW, Chou FC, Cheng CY, Liu CJ (2007) Association of pretreatment thrombocytosis with disease progression and survival in oral squamous cell carcinoma. Oral Oncol 43(3): 283-288.
- Gao J, Zhang HY, Xia YF (2013) Increased platelet count is an indicator of metastasis in patients with nasopharyngeal carcinoma. Tumour Biol 34(1): 39-45.
- Rachidi S, Wallace K, Day TA, Alberg AJ, Li Z (2014) Lower circulating platelet counts and antiplatelet therapy independently predict better outcomes in patients with head and neck squamous cell carcinoma. J Hematol Oncol 7: 65.
- Chen M-H, Chang PM-H, Chen P-M, Tzeng C-H, Chu P-Y, et al. (2009) Prognostic significance of a pretreatment hematologic profile in patients with head and neck cancer. J Cancer Res Clin Oncol 135(12): 1783-1790.
- Shoultz-Henley S, Garden AS, Mohamed ASR, Sheu T, Kroll MH, et al. (2016) Prognostic value of pretherapy platelet elevation in oropharyngeal cancer patients treated with chemoradiation. International journal of cancer 138(5): 1290-1297.
- Pardo L, Valero C, López M, García J, Camacho M, et al. (2017) The prognostic value of pretreatment platelet count in patients with head and neck squamous cell carcinoma. Auris Nasus Larynx 44(3): 313-318.
- Takenaka Y, Oya R, Kitamiura T, Ashida N, Shimizu K, et al. (2018) Platelet count and platelet-lymphocyte ratio as prognostic markers for head and neck squamous cell carcinoma: Meta-analysis. Head and Neck-Journal for the Sciences and Specialties of the Head and Neck 40(12): 2714-2723.
- Baranyai Z, Josa V, Toth A, Szilasi Z, Tihanyi B, et al. (2016) Paraneoplastic thrombocytosis in gastrointestinal cancer. Platelets 27(4): 269-275.
- Lin CY, Kwon H, Rivera GOR, Li X, Chung DJ, et al. (2018) Sex Differences in Using Systemic Inflammatory Markers to Prognosticate Patients with Head and Neck Squamous Cell Carcinoma. Cancer Epidemiology Biomarkers & Prevention 27(10): 1176-1185.
- Eidelman O, Jozwik C, Huang W, Srivastava M, Rothwell SW, et al. (2010) Gender dependence for a subset of the low-abundance signaling proteome in human platelets. Human genomics and proteomics : HGP 2010: 164906.
- Drabsch Y, Ten Dijke P (2012) TGF-beta signalling and its role in cancer progression and metastasis. Cancer metastasis reviews 31(3-4): 553-568.
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, et al. (2003) Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. The Journal of experimental medicine 198(12): 1875-1886.

 Research Article
Research Article