Abstract
Object: Fluorine-19 (19F)-MRI can detect specific cell subsets in-vivo, and infused perfluorocarbon label accumulates in myocardial macrophages in mice with Experimental Autoimmune Myocarditis (EAM). However, the extent of myocardial migration by labeled macrophages versus uptake by resident macrophages is unknown. Here, we tested the feasibility of tracking by 19F-MRI of in-vitro labeled bone-marrow-derived macrophages (BMM) after their adoptive transfer.
Materials and Methods: BMM from CD45.1 mice were 19F-labeled in-vitro with CS-1000 and were then intravenously injected (1-14 × 106 cells) into CD45.2 BALB/c mice with EAM. In-vivo 19F-MRI (9.4T) was performed 2 days later, and 19F content of organ extracts was determined by 19F-NMR spectroscopy, with BMM migration characterized by immunohistochemistry and flow cytometry.
Results: Labeling yielded 1.02 ± 0.50 × 1013 19F-atoms/cell, and a variable invivo 19F-MRI signal was detected in the liver, lungs, and spleen. A faint 19F signal was detectable in some isolated hearts, and the CD45.1 marker was present in a minor fraction of inflammatory cells. Ex-vivo 19F-NMR, immunohistochemistry, and flow cytometry confirmed these in-vivo measurements.
Discussion: 19F-MRI-based cell tracking in the EAM model demonstrates accumulation of intravenously-injected labeled BMM primarily in the liver, spleen, and lungs, with minimal accumulation in the inflamed myocardium.
Keywords: Experimental Autoimmune Myocarditis; Bone-Marrow Macrophage; Perfluorocarbon; 19F-MRI; 19F-NMR
Abbreviations: 19F: Fluorine-19; ANOVA: Analysis of Variance; BMM: Bone- Marrow Macrophages; EAM: Experimental Autoimmune Myocarditis; FBS: Fetal Bovine Serum; IV: Intravenous; PFC: Perfluorocarbon; MRI: Magnetic Resonance Imaging; MRS: Magnetic Resonance Spectroscopy; NMR: Nuclear Magnetic Resonance; OCT: Optimal Cutting Temperature; PBS: Phosphate Buffered Saline; RPMI: Roswell Park Memorial Institute; SPECT: Single-Photon Emission Computed Tomography; SPGR: Spoiled-Gradient Echo; SSFP: Steady State at Free Precession; T: Tesla; TE: Echo Time; TR: Repetition Time; TFA: Trifluoroacetic Acid
Introduction
In recent years, 19F-MRI has emerged as an attractive method to detect perfluoro-carbon (PFC) compounds non-invasively in animal experiments [1,2]. Because 19F background signal is effectively absent in animals and humans, 19F-MRI can unequivocally detect an exogenous 19F compound with high specificity of the signal. As the signal received by an MR volume coil with a homogenous B1 field is directly proportional to the amount of 19F nuclei present in the tissue under experimental conditions allowing uniform nuclear spin relaxation and excitation, the signal can be related to a reference of known concentration, rendering this technique quantitative [3,4]. Moreover, unlike molecular imaging methods based upon positron emission or hyperpolarized carbon-13, non-volatile PFC compounds are not limited by signal decay over time, and the time window for their detection can therefore last several days. Finally, 19F-MRI images can be merged with conventional 1H-MRI images to match the 19F signal with its exact anatomic location in the body and to also correlate it with function or other tissue characteristics.
Accordingly, 19F-MRI has been used successfully to detect and track well-defined cell populations in rodent models of inflammation, including myocardial infarction [5-7], cerebral ischemia [6], pneumonia [8], atherosclerosis [9], arthritis [10], and tumors infiltrated by macrophages [11]. In a recent study, we successfully visualized heart-infiltrating macrophages in-vivo by 19F-MRI in a model of experimental autoimmune myocarditis (EAM) after IV injection of a PFC emulsion [12]. The EAM model is a CD4+ T cell-mediated disease which closely resembles the phenotype of human myocarditis, including its progression toward inflammatory dilated cardiomyopathy [13]. Inflammatory cardiomyopathy, on the other hand, is an important cause of heart failure in young patients and its prevalence is most likely largely underestimated [14]. Cardiac inflammation in EAM typically peaks approximately 21 days after immunization, as assessed by hematoxylin and eosin histological staining of inflammatory infiltrates, followed by a transition to diffuse interstitial fibrosis and end-stage heart failure [15].
Our earlier study demonstrated by immunohistochemistry and flow cytometry the presence of PFC in macrophages within the inflamed myocardium [12]. However, the precise origin of these PFC-labeled macrophages remains unknown since the IVinjected PFC can be taken up by either circulating monocytes or by macrophages which differentiate from heart-infiltrating or resident immature precursor cells in the inflamed myocardium. Several lines of evidence suggest an immunosuppressive role of myeloid cells and macrophages in regulating disease severity and outcome in myocarditis [16,17]. If circulating cells are responsible for a major part of cardiac infiltrates detected by 19F-MRI in the EAM model, it should then be possible in principle to follow the migration of labeled monocytes or macrophages to the inflamed myocardium.
Recent reports have demonstrated that T cells, splenocytes, stem cells, and endothelial cells can be labeled with PFC in-vitro and then re-injected to be tracked by 19F-MRI [4,18-22]. The aim of this study was to determine the feasibility of using this method to track macrophage migration in the EAM model as a proof-ofconcept. Bone-marrow-derived macrophages (BMM) were labeled with PFC in-vitro and re-injected into EAM mice in the early phase of inflammation, before the peak, for cell tracking by 19F-MRI. 19F-MRI was performed on the heart as well as the lungs, spleen and liver to best characterize the fate of the injected PFC-labeled BMM, and the results were confirmed with ex-vivo 19F-NMR spectroscopy, immunohistochemistry, and flow cytometry experiments.
Materials and Methods
Animals
Mice were maintained under specific pathogen-free conditions. EAM was induced in 52 male BALB/c (CD45.2) mice by subcutaneous injections of αMyHC peptide (alpha myosin heavy chain, Ac- RSLKLMATLFSTYASADROH; Caslo, Lyngby, Denmark) emulsified 1:1 with complete Freund’s adjuvant (Difco, Franklin Lakes, NJ) as previously described [13]. Immunization with the peptide was performed at days 0 and 7, following the in-vivo protocol timeline depicted in Figure 1. In the EAM model, immunization results in acute myocarditis that peaks ~21 days after immunization. Inflammation resolves slowly thereafter, whereas the number of cardiac fibroblasts progressively increases [15].
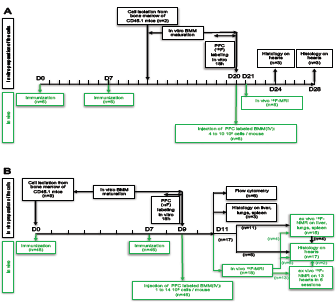
Figure 1: In-vitro and in-vivo protocol description. Overview of the time scale of the different experimental procedures. In-vitro procedures are depicted in black and in-vivo procedures are depicted in green.
0(A) “Long time” protocol. In-vitro: Cell preparation. Bone marrow cells were isolated from femurs of CD45.1 mice (n=2) and cultured as described in the Materials and Methods section. In-vivo: At day 0
(D0) and D7, BALB/c mice (n=6) were immunized to induce EAM. At D20 the mice received an IV injection of 19F-labeled- BMM (from 4 to 10 × 106 cells /mouse). All the mice were analyzed by in vivo 1H- and 19F-MRI at D21 and also either at D24 (n=3) or D28 (n=3). These mice were euthanized immediately after the MRI session and their hearts were analyzed by histology.
(B) “Short time” protocol. In-vitro: Cell preparation. At D0 bone marrow cells were isolated cultured as before (9 mice). In-vivo: At D0 and D7, BALB/c mice (n=46) were immunized as before. At D9 all the mice received an IV injection of 19F-labeled-BMM (from 2 to 10 × 106 cells /mouse). Mice were scanned and / or analyzed 36 hours after BMM injection.
Bone Marrow Macrophages Isolation
CD45.1-positive BALB/c mice (n=11) were used as donors to identify the transferred cells in the CD45.2 recipients. Briefly, femurs and tibiae were removed, the surrounding muscle tissue was detached, and the bones were kept in Roswell Park Memorial Institute (RPMI) 1640 media. Marrow was flushed out with RPMI 1640 using a syringe and passed through a 70 μm nylon cell strainer (BD Biosciences) to remove debris. Cell suspensions were centrifuged and resuspended in complete RPMI 1640 medium supplemented with penicillin-streptomycin, 50 mM betamercaptoethanol, fetal bovine serum (FBS) and sodium pyruvate. Cells were seeded in 100 mm diameter low-adherent bacteriological petri dishes at 5 × 106 cells per dish in 12 ml of complete RPMI medium containing L929 conditioned medium (30:20). Cells were cultured 7 days.
BMM Labeling with 19F Nanoemulsion
BMM were labeled in-vitro with the 19F-based agent Cell Sense (CS-1000). Cell Sense is an aqueous colloidal suspension (“nanoemulsion”) of PFC, having a total fluorine content of 145 mg/ mL (Celsense Inc., Pittsburgh, PA, USA). The average nanoemulsion droplet size is 180 nm. It is formulated with excipients that facilitate entry of the PFC into all cell types, regardless of their ability to phagocytose. The PFC molecule used in Cell Sense is stable at low pH [1]. PFC was added to the cell culture medium at a concentration of 10 mg/mL and incubated for 18 h. After this incubation period, the cells were washed three times with phosphate buffered saline (PBS) (to eliminate free Cell Sense) and then counted after gentle harvesting with a cell scraper.
In-vitro 19F-NMR Spectroscopy of Labeled Cells
In order to measure the mean 19F content present in the cells after labeling, quantitative 19F NMR measurements were performed in lysed cell pellets. A known number of labeled cells (~3 × 106) were spun down and resuspended in 250 μl of 1% (v/v) Triton X-100 in PBS to lyse the cells. The cell lysate was mixed with 250 μl of a calibrated 19F reference solution, trifluoroacetic acid (TFA) at 0.1% v/v in D2O and placed in a 5 mm borosilicate NMR tube. The 19F NMR measurements were performed using a Bruker AVANCE III HD 400 MHz (9.4 T) NMR spectrometer equipped with a BBFO probe (Bruker BioSpin AG, Fällanden, Switzerland). The average 19F-fluorine content per cell was calculated from the ratio of the integrated areas of the TFA and PFC 19F spectra, normalized to the total cell number in the lysate. The PFC 19F spectra, acquired with 256 scans (32,000 points, 15 kHz spectral width and 5 s pre-acquisition delay) and processed with 5 Hz line broadening, contain several peaks, with the most prominent one located at -93 ppm. This peak was compared to TFA at -75 ppm for quantitative calculations.
BMM Transfer into EAM Mice
PFC-labeled BMM (1 to 14 × 106) suspended in 250 μl NaCl solution were injected IV into EAM mice either at day 20 or at day 9 (Figure 1).
1H and 19F MRI
At day 11 (36 h post BMM injection), mice (n=18) were anesthetized with intraperitoneal injection of ketamine : medetomidine (75 mg/kg : 0.1 mg/kg). This anesthetic combination was chosen to avoid any 19F background signal from isoflurane, which accumulates in the fat pads of mice [23,24]. Body temperature was monitored with a rectal probe (SA Instruments, Stony Brook, NY) and kept constant at 37.0°C using tubing with circulating warm water. The animals were placed over a custom-designed 18-mm diameter quadrature surface coil tunable to both the 1H and 19F frequencies (400.2 and 376.6 MHz, respectively) [12]. Scanning was performed in a 31 cm horizontal-bore 9.4 T magnet (Magnex, Abingdon, UK) equipped with 400 mT/m gradient coils (Magnex, Abingdon, UK) and a VNMRS console (Varian, Palo Alto, USA).
For in vivo 19F-MRI, a stack of 3 to 5 prospective ECG-triggered short-axis 1H images of the heart was acquired with a gradient echo sequence (repetition time (TR) 11.1 ms, echo time (TE) 1.9 ms, signal averages 4, matrix 128×128, field of view 30×30 mm2, slice thickness 2 mm, spectral width 78,125 Hz, total acquisition time ≈5 min). Next, a stack of non-triggered 19F images was acquired with the same view plan as the 1H images using a fast spin echo (TSE / RARE) sequence (TR 500 ms, TE 3.7 ms; echo-train length 4, signal averages 480, matrix 32x32, field of view 30×30mm2, slice thickness 2 mm spectral width 156.25 kHz, total acquisition time 32 min). The 19F offset frequency was calibrated from unlocalized spectra (40 μs hard pulse, TR 1 s, 128 scans).
19F images were processed in MATLAB with a 6-pixel Gaussian smoothing filter to reduce noise, then interpolated to match the background axis 1H image resolution and thresholded to display signals greater than 4-fold the standard deviation of the noise. Noise in the images was defined by selecting a 10 x 5 mm region in the field of view that did not cover the mouse body. 1H and 19F images corresponding to the same view plan were overlaid. In a subset of experiments (n=13), ex vivo 19F-MRS was performed on intact excised hearts placed in 1.8 ml microcentrifuge tubes filled with PBS buffer. One, two or four hearts in tubes were placed on the quadrature surface coil, and all hearts were scanned in 6 sessions with a pulse-acquire sequence (40 μs hard pulse, TR 1 s, 1024 or 2048 scans, spectral width 20161.3 Hz, 4129 points).
Organ Collection
The mice were euthanized to collect the organs after MRI scanning. Thirteen of the hearts were immediately analyzed by 19F-MRS prior to being embedded in optimal cutting temperature (OCT) compound for immunohistochemistry. The liver, lungs, and spleen of 8 unscanned mice were also collected and divided for FACS analysis and immunohistochemistry, the latter set embedded in OCT; their bone marrow was also collected. The hearts of 10 other mice were also embedded in OCT. The liver, lungs, and spleen of 11 unscanned mice were collected for ex-vivo 19F NMR spectroscopic analysis.
Ex-vivo 19F-NMR Spectroscopy of Excised Organs
The 19F NMR measurements were performed on the homogenates prepared from liver, lungs and spleen in 1% (v/v) Triton X-100 in PBS. The cell lysates were then mixed with 250 μl of 0.1% (v/v) TFA in D2O (calibrated 19F reference solution) and placed in a 5 mm borosilicate glass NMR tube. Spectra were acquired as described above in the “In-vitro 19F-NMR spectroscopy of labeled cells” section.
Immunohistochemistry
OCT-embedded snap frozen tissues (heart, liver, lung and spleen) were sectioned in a cryostat at 6 μm thickness. Slides were air dried and fixed in acetone for 10 min at room temperature. Immunohistochemistry was performed on a Leica Bond Max according to the manufacturer’s guidelines. The primary antibody, mouse anti-murine CD45.1-FITC (BD Cat. 553775, dilution 1:500), was linked with the secondary antibody, Rabbit anti-FITC (Serotec Cat. 4510-7804, dilution 1:500) and detected with Polymer anti- Rabbit AP-Refine-Kit (Cat. 9390) from Leica.
Flow Cytometry
Single cell suspensions were prepared from bone marrow, liver, lungs and spleen using a cell strainer (70 μm) and RPMI medium for flow cytometry. After centrifugation, the pellets were resuspended in FACS buffer containing alexa647-conjugated anti-CD45.1 or IgG negative control for immunostaining (30 min at 4°C in the dark). Cells were washed with PBS and analyzed on a FACS LSRII (BD Biosciences, San Diego, CA) using BD FACS Diva software.
Statistical Analyses
Values are given as mean ± standard deviation. Analyses of differences between groups were performed using one-way analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons. Normality was evaluated with the Shapiro- Wilk test.
Results
In-vitro 19F-Labeling of BMM
The uptake after 18 hours of incubation with the PFC agent was quantified by 19F NMR and yielded 1.02 ± 0.50 × 1013 19F atoms/cell (n=6). Cell viability was assayed immediately after labeling (18 h) by Trypan blue exclusion assay to evaluate the potential cytotoxicity due to labeling. The fraction of dead cells after PFC incubation was comparable to incubation in the absence of PFC (approximately 0.5% for both), indicating that the PFC agent safely labeled these cells in-vitro without significantly affecting their viability.
Detection of 19F Signal by 19F-MRI
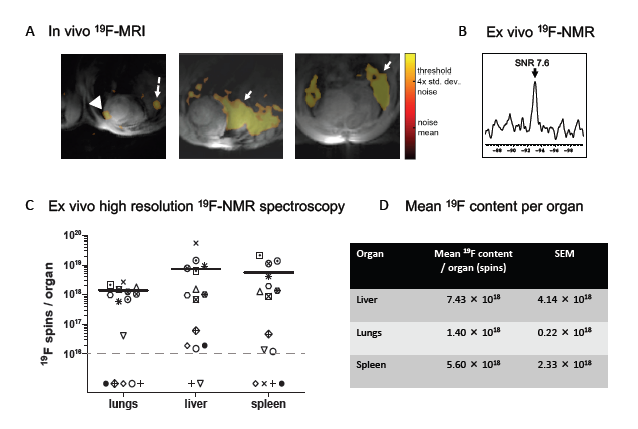
Protocols with early (day 9) and late (day 20) 19F-labeled cell injection and cardiac follow-up 19F-MRI studies were applied (Figure 1). The late injection protocol followed a similar timeline as in our previous study where PFC was directly IV administered into EAM mice [12]. In 6 mice, PFC-labeled BMM were injected IV at day 20 after the first immunization of recipient mice, and invivo cardiac 19F-MRI was performed at days 21, 24 and 28. Under these conditions, no 19F-signals were detected in the heart by 19F-MRI (data not shown). Given that heart-infiltrating T cells and monocytes in the EAM model appear as early as 7-10 days after the first immunization, we decided to change the time course of our experiments. In the early series, 19F-labeled BMM were injected at day 9, and in-vivo cardiac 19F-MRI was performed at day 11. Although 19F signal of variable intensity was detected in all animals by spectroscopy, the MRI signal detected in the heart was weak or absent (Figure 2A, arrowhead) and the majority of the 19F-signal was identified in the lungs (solid arrow) and the liver (dashed arrow).
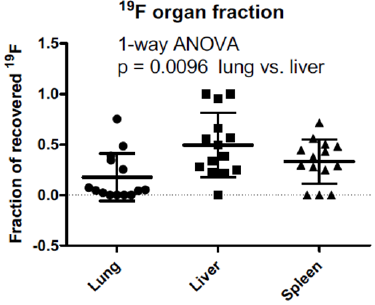
These in-vivo data were confirmed by post-mortem analyses of the organs. Hearts were analyzed ex-vivo by 19F-MRS (n=13), and a faint spectral peak was detected within the combined cardiac tissue of 4 animals (Figure 2B). No 19F ex-vivo heart signal was detected in the other animals. The 19F content of the other organs (liver, lungs, spleen) was determined by 19F-NMR in tissue homogenates. Most of the organs analyzed contained variable amounts of 19F (Figures 2C & 2D), reflecting the variable 19F-MRI signal seen in vivo. The fraction of 19F recovered in the organs was significantly higher in the liver than the lungs (p = 0.0096, Figure 3), even though the liver 19F content was below the limit of detection in two unscanned mice injected with weakly-labeled BMM (6.49 × 1011 and 2.37 × 1011 19F atoms/cell).
Figure 2: In-vivo and ex-vivo 19F-detection. (A) Representative in-vivo short-axis fused 19F and 1H images. 1H-signal appears in grey, 19F-signal in orange, and the heart, liver and lungs can clearly be identified. The highest signal was detected in the liver (dashed arrows) and in the lung (solid arrows). Heart signal is weak (arrowhead) and patchy, in agreement with a patchy infiltration of the EAM heart previously reported [12]. (B) Ex-vivo 19F-MRS of four intact hearts showing a PFC peak. (C) and (D) 19F content in the liver, lungs and spleen determined by ex-vivo 19F-NMR spectroscopy of homogenate. (C) Each symbol corresponds to a different mouse; the black horizontal lines indicate the mean value for samples with signal. Samples without any 19F signal appear below the dotted line indicating the limit of detection by 19F-NMR. (D) Table with the mean 19F-atom content per organ.
Figure 3: Fraction of recovered 19F label in lungs, liver and spleen. The 19F content of each organ is divided by the sum of the 19F content of the three organs in a mouse. Samples with 19F below the limit of detection by NMR were assigned the value of zero. The liver had on average the highest fraction of recovered 19F, which was significantly higher than the lung fraction.
Immunohistochemistry
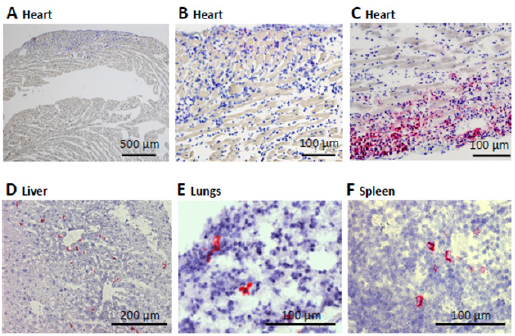
The presence of CD45.1 donor cell infiltrates was identified by immunohistochemistry in 12 out of 17 hearts, including 10 mice that underwent the same treatment but were not scanned. CD45.1 infiltrates were also found by immunohistochemistry in the liver, the lungs, and the spleen of the 3 mice analyzed (Figure 4).
Figure 4: Immunohistochemistry CD45.1 in pink. ABC: Heart sections, D: Liver; E: Lungs; F: SpleenImmunohistological analysis of inflammatory cell infiltration. Representative CD45.1 staining to identify BMM donor cells (red, with blue hematoxylin staining of cell nuclei) in the CD45.2 recipients. (A) and (B) are representative examples of a heart with a low myocarditis score (1) and no cardiac infiltration by donor BMM cells. (C) illustrates weak cardiac infiltration by donor BMM cells in a heart with myocarditis score of 2. The mice in (A, B and C) were injected with the same labeled BMM preparation. CD45.1 positive infiltrates can be observed in liver (D), lungs (E), and spleen (F) sections. The scale is indicated on each image.
Flow Cytometry
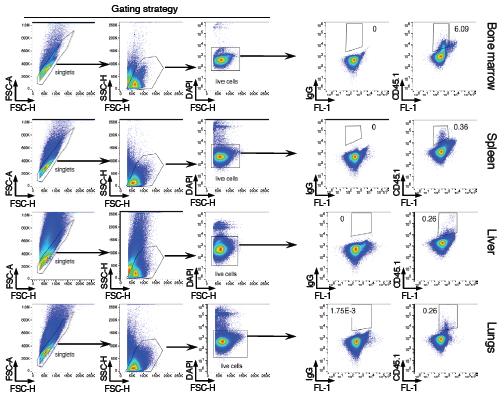
To confirm the immunohistochemistry results, flow cytometry of the CD45.1 marker was used to follow the distribution of the donor BMM in the different organs (Figure 5). Flow cytometric analysis confirmed the presence of the injected CD45.1 cells in the organs, including bone marrow (6.2 ± 0.2 % of the gated cells), spleen (0.4 ± 0.03 %), liver (0.2 ± 0.04 %) and lungs (0.4 ± 0.2 %).
Figure 5: Flow cytometry analysis. Cells were isolated from the indicated organs and analyzed by flow cytometry. Arrows in the left panel indicate gating strategy. Plots in the right panel show CD45.1 analysis on gated populations. Isotype IgG were used as negative control. FL-1 represents empty channel. Numbers indicate percentage of cells in the adjacent gates. Data are representative of 8 mice.
Discussion
The main findings of the current study can be summarized as follows: 1) BMM were successfully labeled in-vitro by PFC, yielding (1.02 ± 0.50) x 1013 19F atoms/cell while preserving their viability. 2) As a proof-of-concept, IV-injected 19F-labeled mature BMM can be tracked in the EAM model by 19F-MRI, demonstrating their accumulation primarily in the liver, spleen and lungs, while accumulation in the inflamed myocardium is minimal despite the development of myocarditis. 3) The distribution of 19F-labeled BMM revealed by in-vivo 19F-MRI was confirmed by ex-vivo 19F-NMR spectroscopy, immunohistology, and flow cytometry.
Detectable Migration of IV-Injected BMM into Extra- Cardiac Tissue
The presence of the PFC-labeled BMM in the liver, lungs, and spleen was readily apparent by 19F-MRI and confirmed by ex-vivo 19F-NMR spectroscopy. This pattern of accumulation is reminiscent of the one previously reported for labeled BMM tracked by singlephoton emission computed tomography (SPECT) and T2*-weighted MRI, where the cells were primarily located in the liver and spleen after initially being found mainly in the lung [25]. The similar pattern suggests that these two different labeling methods do not affect the BMM migration behavior, but a functional effect due to the labeling cannot be ruled out.
Weak Invasion of the Myocardium in the EAM Model by BBM
In earlier studies, direct IV injection of PFC nanoemulsions successfully labeled macrophages in inflamed myocardium of the EAM model [12] and in other models of myocarditis [26]. In the current study, however, PFC-labeled BMM, when injected IV, did not migrate to the site of inflammation in the heart at a high level and were rarely detectable there by in-vivo 19F-MRI or ex-vivo 19F-NMR spectroscopy. In-vivo 19F-MRI and ex-vivo 19F-NMR spectroscopy detected a 19F signal in the heart in 2 out of 18 and 1 out of 6 experiments, respectively. However, in-vivo and ex-vivo signals were not detected in the same mice, and the apparent in-vivo 19F cardiac signal may be due to partial-volume effects from the neighboring lung and liver. In an earlier study [18], the same in-vivo 19F-MRI experimental setup yielded a threshold for detection of 1.5 × 1017 19F-atoms per 0.63mm3, which translates to approximately 8500 cells/μl (assuming a labeling of 1013 19F-atoms/cell) with the 1.76 mm3 voxel size used here. Nonetheless, a substantial fraction of the injected labeled cells - on the order of 1% or more - would have to be concentrated in a 9 mm3 volume (assuming five adjacent voxels) of the myocardium in order to be reliably detected.
This may partially explain the infrequent and inconsistent detection of myocardial 19F signal despite the presence of CD45.1- positive transferred cells in the myocardium two-thirds of the time (i.e. in 12 out of 17 hearts by immunohistology). In the case where a 19F signal was detected ex vivo (Figure 2B), the signal is estimated to correspond to ~2 x 1017 19F atoms, assuming the same coil loading and spatial distribution of 19F in the phantom used for calibration and was likely to diffuse to be detectable by imaging. A number of factors must be considered regarding the origin and fate of PFClabeled macrophages in the EAM mouse heart in the interpretation of these results. Depending on the particular model used, there is evidence for both local macrophage differentiation or proliferation in the inflamed myocardium [27-29] and also macrophages derived from recruited blood monocytes [30].
Reports from our laboratories clearly indicate an important role of heart-infiltrating bone-marrow-derived CD133-expressing immature monocyte-like precursor cells as a potential source of both myofibroblasts and macrophages in the inflamed heart [28,31,32]. On the one hand, the uptake of IV-infused PFC by phagocytic cells in the circulation, followed by their migration to the inflamed myocardium, provides a good rationale for the myocardial accumulation of the 19F signal in previous myocarditis studies [12, 26]. Bönner et al. have shown the ability of blood-derived leukocytes to take up PFC [33], which would support this route of myocardial PFC entry. On the other hand, it is possible for cells already present in the heart to take up infused PFC in quantities sufficient to be imaged. For example, Ding et al. demonstrated infiltration of the inflamed myocardium in an ischemia-reperfusion model not only by blood-derived monocytes but also by epicardium-derived cells (EPDC) [34].
These EPDC displayed strong endocytic activity to take up the IV-injected PFC emulsion and infiltrated the inflamed myocardium in a time-dependent manner, such that they represented the main source of 19F-labeled cells 3 days after the ischemia-reperfusion event. If BMM migration is not affected by labeling, the present results would indicate that the injected BMM are unable to effectively infiltrate the heart in large numbers. This would be consistent with the view that accumulation of macrophages within the inflamed heart results either from differentiation of recruited or resident precursor cells or from proliferation of those already present. Nevertheless, to better test this mechanism of PFC accumulation in the inflamed myocardium would require a separate protocol to track PFC-labeled monocytes in the murine EAM model.
Limitations
The techniques used in this study result in a number of limitations. One important limitation of the PFC-labeling technique is the considerable variability of the achieved loading, ranging from 2.37 × 1011 to 5.34 × 1013 19F-atoms/cell, which translated into the variable number of injected BMM cells, ranging from 1 to 14 × 106 per animal. Overall, less 19F was administered to the mice as PFC-labeled BMMs than in our previous myocarditis study with infused PFC, and this may limit sensitivity. Despite the >10- fold higher average per-cell 19F content compared to prior studies using IV-transferred PFC-labeled cells [18,20], there still was not sufficient sensitivity to detect the label in the target tissue in vivo. Additionally, the in vivo MRI and ex vivo MRS were performed with a surface coil, which provides high sensitivity but makes absolute quantitation of the 19F more difficult. To better quantitate the in vivo 19F signal, a volume coil could be used with a reference standard in the field of view.
The sensitivity of the 19F imaging sequence could also be further improved with a lower acquisition bandwidth, or by using a spoiled-gradient echo (SPGR), steady state at free precession (SSFP)-type method instead [21]. Although the procedure to differentiate bone-marrow derived macrophage precursor cells with L929-conditioned medium is a well-accepted method to generate a relatively uniform population of mature quiescent BMM in high yield [35], the phenotype of the transferred cells found in the liver, lungs, spleen, heart and bone marrow was not characterized beyond the presence of the CD45.1 marker. While PFC labeling did not affect BMM pre-infusion viability, further study is needed to understand whether it has any effect on cell migration to the inflamed myocardium in the EAM model.
Conclusion
BMM were successfully labeled in-vitro with PFC and tracked in-vivo by non-invasive 19F-MRI. In the EAM model, non-invasive 19F-MRI reliably demonstrates accumulation of IV-injected mature BMM primarily in the liver, spleen, and lungs, with little migration to the inflamed myocardium. Thus, this technique has the potential to non-invasively track the in-vivo migration of specific cell types in the EAM model. These findings suggest that either resident inflammatory cells, macrophages differentiating from heart-infiltrating immature precursors represent the main source of differentiated macrophages accumulating in the heart in myocarditis and point the way to further studies that directly address these questions.
Declarations
Ethics Approval and Consent to Participate
All animal procedures were approved by the institutional ethics committee and performed in accordance with an authorization for animal experimentation issued by the local regulatory agency: le Service de la Consommation et des Affaires vétérinaires du Canton de Vaud (SCAV, Epalinges, Switzerland).
Consent for Publication
Not applicable.
Availability of Data and Materials
The datasets used and analyzed during the current study are available from the corresponding author (J.S.) on reasonable request.
Competing Interests
The authors declare that they have no competing interests.
Funding
This work was supported by the Swiss National Science Foundation grants 310030_163050 and PZ00P3_154719, as well as a grant from the Swiss Heart Foundation to J.S. (2015).
Authors’ Contributions
Study Conception and Design: Gonzales, Schwitter, Helm, Eriksson
Acquisition of Data: Gonzales, Yoshihara, van Heeswijk, Mieville, Blyszczuk, Kania
Analysis and Interpretation of Data: Gonzales, Yoshihara, Mieville, Blyszczuk, Kania
Drafting of Manuscript: Gonzales, Yoshihara, Schwitter, Eriksson
Critical Revision: van Heeswijk, Blyszczuk
Acknowledgment
We are grateful to Carola J. Romero, Corina M. Berset, Anne- Catherine Clerc for their technical assistance.
References
- Spanish flu. Wikipedia
- Kolte IV, Skinhöj P, Keiding N, Lynge E (2008) The Spanish flu in Denmark. Scandinavian Journal of Infectious Diseases 40(6-7): 538-546.
- Hindhede M (1920) THE Effect of Food Restriction During War on Mortality in Copenhagen. JAMA 74(6): 381-382.
- (1919) Hindhede M Ugesk. Laeger 81:183 Abstr JAMA 72: 1198.
- Karström H (1978) Oikea ravinto terveytemme perusta. Kirjatoimi offset Tampere pp. 94-95
- Chittenden RH (1905) Physiological Economy in Nutrition. Heineman, London 73: 328-330.
- Hindhede M (1909) Min reform. Stockholm, PA Norstedt & Söners Fö
- Töysä T (2019) Monthly Variation in Finnish Total Mortality during 1891-2017, Temperature and Discussions. Biomed J Sci & Tech Res 14(4): 1-4.
- McGhee P (1918) Influenza 1918” in Wikipedia. Spanish flu, refer. [41].
- BENCKO V (2004) Lethality rate. Hygiene and Epidemiology : Selected Chapters (2nd), Prague. 2004. ISBN 80-246-0793-X, in Wikilectures.
- First World War. Wikipedia.
- Koivistoinen P (1980) Mineral Element Composition of Finnish Foods: N, K, Ca, Mg, P, S, Fe, Cu, Mn, Zn, Mo, Co, Ni, Cr, F, Se, Si, Rb, Al, B, Br, Hg, As, Cd, Pb and Ash. Acta Agriculturae Scandinavica 22: 165-171.
- Koivistoinen P (2011) Personal communication.
- Uusitalo U, Pietinen P, Leino U (1990) Food and Nutrient Intake Among Adults in East and Southwest Finland - A Dietary Survey of the Finmonica Project in 1982, National Public Health Institute. Helsinki, Finland 1987. Kansanterveyslaitoksen julkaisuja - Publications of the National Public Health Institute.
- (2020) Energy density of food. Specific energy.
- (2009) SFK.Online.web: www.sfk-online-net.
- Energy content of biofuel.
- White A, Handler P, Smith EL (1968) Priciples of Biochemistry. Chapter 48: 1015. In White A, Handler P, Smith EL (Eds.), McGraw-hill book company/Kōgakusha company, LTD pp. 93.
- Elin RJ (1994) Magnesium: the fifth but forgotten electrolyte. Am J Clin Pathol 102(5): 616-622.
- Töysä T (2018) Regional associations of CHD and musculoskeletal morbidity with environmental and geographic factors – e.g. Ca, Mg, Si, Sn. Biomed J Sci & Tech Res. 4(2): 3797-3803.
- Parantainen J, Tenhunen E, Kangasniemi R, Sankari S, Atroshi F (1987) Milk and blood levels of silicon and selenium status in bovine mastitis. Vet Res Commun 11(5):467-477.
- Pilon C, Soratto RP, Broetto F, Fernandes AM (2014) Foliar or Soil Applications of Silicon Alleviate Water-Deficit Stress of Potato. Crop Ecology & Physiology. 106(6): 2325-2334.
- Rutella GS, Solieri L, Martini S, Tagliazucchi D (2016) Release of the Antihypertensive Tripeptides Valine-Proline-Proline and Isoleucine-Proline-Proline from Bovine Milk Caseins during in Vitro Gastrointestinal Digestion. J Agric Food Chem 64(45): 8509-8515.
- Valkonen T, Martikainen P (1990) Development of mortality from ischaemic heart disease in subgroups of the population in Finland. Sosiaalilääketieteellinen Aikakauslehti. Journal of Social Medicine 27: 273-288.
- Toysa T, Hanninen O (2016) Compliance of Finnish Male CHD and Total Mortality with Soil Fertilization in 1957-1990. J Agriculture 2(1): 013.
- Pennington JA (1991) Silicon in foods and diets. Food Addit Contam 8(1): 97-118.
- (2012) Nordic Nutrition Recommendation. Integrating nutrition and physical activity. (5 Edn.), Nord 014:002.

 Research Article
Research Article