Abstract
This paper reports the synthesis of two Schiff base ligands, L1Hand L2H obtained from the reflux-reaction of 2-hydroxy-1-naphthaldehyde (HNA) with 2-aminopyrimidine (AMP) and 2-amino-4,6-dihydroxypyrimidine (ADHP) separately. The synthesized ligands were characterised via analytical and spectroscopic methods; and evaluated for environmental mob up potentials through solvent extraction process. The latter showed excellent extractive ability of the ligands on both Ni2+ and Cu2+ ions in basic and acidic media with the best obtainable at pH of 9. The electronic data indicated that the Schiff bases assumed no geometry but displayed transitions involving electrons amid intra ligand orbitals. The infrared (IR) spectra data revealed basic functional moieties within the ligands which were not found in the starting materials confirming the formation of Schiff bases.
Introduction
Transition elements are naturally obtainable within the earth curst but constitute large number of contaminants to the environs owing to their non-biodegradable nature [1]. The latter known as heavy metals, in both terrestrial and aquatic environments, has been identified as leadingserious contaminants due to their toxicity (Peralta et al., 2009); gaining entrance and has become concentrated in plants, animals and human tissue through inhalation, diet, and manual treatment [2]. Common sources through which the latter are discharged into the surroundings includes industrial wastes discharge, fertilizers, car discharges, Pb-acid batteries, mining activities, aging facility infrastructure, etc [3] and floating-plastics within the oceans [4]. Metallic species are found everywhere, for example lead (Pb), arsenic (As) and cadmium (Cd) are available in kids’ dolls by levels beyond restrictive stages. While Pbis adopted as a color enhancer in toys, stabilizer or as a resistant-eroding agent, Cd is utilized as preservative to enhance the luster of doll necklaces. Arsenic is believed to be utilized in association with coloring dyes [5]. Heavy metals can bind to vital cellular parts, like structural proteins, enzymes, and nucleic acids, and interfere with their functioning [6]. Indications and effects differ in line with metallic ions, as well as the dosage concerned. Continuing contact with toxic metals leads to cancer, circulatory, dominant and outlying nervous system effects. In humans, significant metallic poisoning is usually cured by the use of chelate assemblages. These significant metals are also essential and needed at low concentration for human health. They’re not required in lots of abundance within the body and in the environment due to their noxious nature [2]. There are several processes adopted for the elimination of metallic species within the environ. Carbon-based compounds with various moieties have been applied in the elimination of metallic species from the environment. Schiff bases bearing functional groups are reported as excellent chelators and good removal agents of heavy metals. The latter are considered products of condensation reaction amid primary amines and carbonyl compounds [7]. Also, Schiff bases are utilized as intermediates in carbon-based synthesis, catalysts, corrosion inhibitors and chemical compound stabilizers [8]. A search through literature shows that synthesized Schiff bases resulting from HNA with AMP and ADHP have not been unutilized in the removal of heavy metals within the environments. Hence this work is targeted to design, synthesis and characterize novel aminopyrimidine organic frameworks as potential environmental mobup agents’ [9,10].
Experimental
Materials
The chemicals used for the synthesis were supplied by Sigma- Aldrich and British Drug Houses (BDH) Limited.They include [Ni2+(CH3CO2)2.4H2O], [Cu2+(CH3CO2)2.2H2O], HNA, AMP, ADHP, acetic acid, ethyl acetate, ammonium solution and ammonium chloride.
Physical Measurements
The IR spectra of the ligands were obtained on PERKIN ELMER FT-IR SPECTRUM BX SPECTROPHOTOMETER using KBr disc in the range 450-4400cm-1. The UV-Vis spectra of the ligands were recorded on PERKIN ELMER LAMBDA 25 UV/VISIBLE SPECTROPHOTOMETER. The elemental (CHN) analysis of the synthesized compounds was evaluated on an Elementary; Vario EL Cube setup at the Nelson Mandela Metropolitan University, South Africa.Solvent extraction was determined by AAS (Sola Thermo Elemental Atomic Absorption Spectrometer SGST 71906).
Synthesis of L1H Ligand:
A combination of equimolar quantity of HNA and AMP were dissolved in dry methanol (30mL). The reaction mixture was refluxed on a magnetic-stirrer hotplate for 6 h after drop-wisely addition of about 3mL of glacial acetic acid for catalysis. The precipitates separated on cooling in ice, filtered under gravity but were recrystallized from methanol to give L1H ligand. The product was stored in the desiccators over silica gel before use.
Synthesis of L2H Ligand
A mixture of HNA and ADHP was mixed with small volume of dry methanol (30mL) in stoichiometric ratio. The solution containing the mixture was refluxed on a magnetic-stirrer hotplate for 6 h after adding 3mL of glacial acetic acid to serve as catalyst, drop-wisely. As it cools, using ice the precipitates was separated and filtered under gravity. Recrystallization process was carried out to give L2H ligand. The desiccator was used to store the product over silica gel before use.
Methodology (Extraction Process)
Buffer solution prepared by mixing ammonium chloride (NH4Cl) and ammonium solution (NH4) with equi-molar amount of distilled water was adopted and used at pH of 5,7 and 9 respectively. A combination of each ligand, metallic salt, chloroform and methanol were mixed together, dissolved in the buffer solution at the different pH and agitated. The latter was separated into two distinct layers using separator funnel. The reaction was carried out in doublets. All measurements were taken as 1.5 x 10-3 and 4 x 10-4 for the metallic salts and the ligands.
Results and Discussion
Synthesis
The Schiff base ligands were acquired through condensation reflux of HNA with AMP and ADHP in molar ratio of 1:1 separately. The formation of the Schiff base was confirmed by UV-vis, FTIR, melting point and solubility test analysis. The ligands were stable at room temperature.
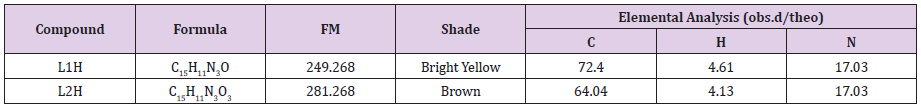
Elemental Analysis and Solubility
The synthesized ligands exhibited various shades of color. The L1H and L2H ligands displayed bright yellow and brown shades singly. The L1H ligand was soluble in EtOH, CH2Cl2, CHCl3, DMSO, DMF and slightly soluble in MeOH, and CH3NO2 whileL2H ligand was soluble in DMSO, DMF and slightly soluble in MeOH, EtOH, CH2Cl2, CHCl3 but insoluble in H2O and CH3NO2. The analytical data and physical properties of the ligands are presented in Table 2. Obtained data were consistent with the calculated results.
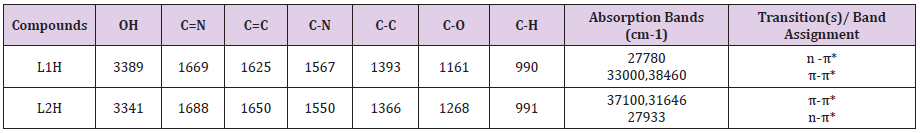
FT-IR Spectra
The band at 3389cm-1 in L1H ligand spectrum was ascribed to v(OH) vibration. The band at 1669cm-1 was allotted to the v(C=N) band. The band associated with v(C=C) stretching was detected at 1625cm-1 . The band at 1567cm-1 as well as1393cm-1 was allocated to v(C-N) and v(C-C) vibrations. The L1Hspectrum gave an absorption band at 1161cm-1 and 990cm-1 which were consistent with v(C-O) and v(C-H) separately. The band at 3341 cm-1 in L2H spectrum was ascribed to v(OH) vibration. The band at 1688cm-1 was assigned to the v(C=N) band. The v(C=C) stretching vibration was detected at 16250cm-1 . The band at 1550cm-1 and 1366cm-1 were apportioned to v(C-N) and v(C-C) vibrations. The L2H ligand spectrum gave an absorption band at 1268cm-1 and 991cm-1consistent v(C-O) and v(C-H).
Electronic Studies
The absorption spectrum of the L1H ligand gave two peaks within the UV region at 38460cm-1 and 27780cm-1 which remained apportioned to π→π* and n→π* transitionssingly. Also, the electronic spectrum of the L2H ligand exhibited two bands at 31646cm-1 and 27933cm-1 due to π→π* and n→π* transitions separately (Tables 1-3).
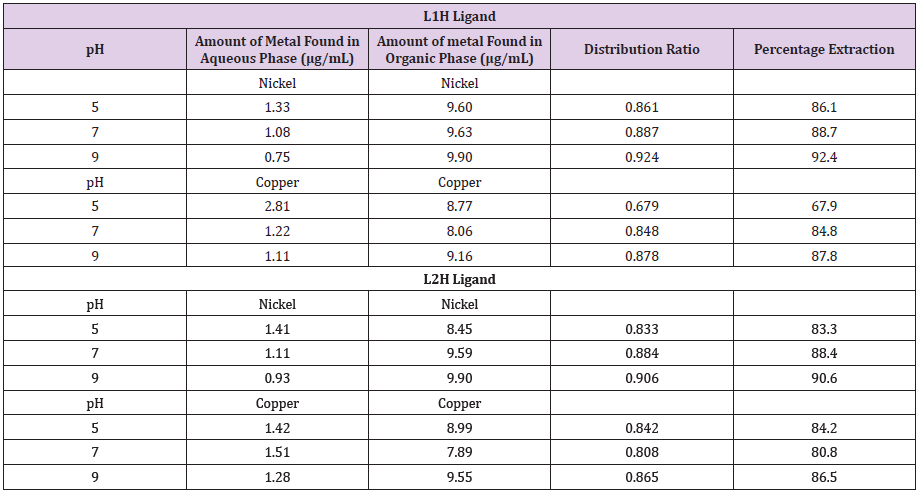
Solvent Extraction Studies
The ligands reacted with ionic copper and nickel on adding the L1H and L2H to the aqueous phase forming colored metallic compounds. While the Cu2+ solution adopted reddish-brown color, the Ni2+ solution gave greenish shade (Table 4). The ligand’s metal ion abstraction ratios too examined with the estimation of amount of Ni2+ and Cu2+ absorbed in aqueous phase at various pHs. The results obtained after the extraction process were given in terms of extraction ratio R(%) and partition coefficient D using the following expressions:

where [Maq] is the quantity of M+ detected in aqueous phase and [Morg] is the amount of metal found in organic phase. The level of separation stood evaluated via separation factor Sf, taken as the ratio of distribution for Ni ion to the distribution ratio for Cu ion:

The extractability as well as selectivity of M2+ ions was assessed as a function of pH. The pH of the aqueous solution prior to extraction stood varied at 5, 7 and 9 by using solutions of NH4Cl (0.1 M) and NH4 (0.1 M).
The most noteworthy extractability of Cu2+ was accomplished at pH 9. The partition factor of Ni2+ to Cu2+ at aqueous solution of pH 9 for L1H and L2H ligand were 1.1 making the integrated ligand anions a reduced reagent in that division of Ni and Cu ions in solution. The dissolvable extraction procedure was as follows:

Where XL=the extractive reagent, n=number of moles, Y=Cu, and Ni ions. The subscript (aq) and (org) mean the aqueous and organic phrasessingly. In the extraction process, a compound of stoichiometric formulation Cu(II) and Ni(II) were obtained in the natural organic phase freeing n moles of X+1 particles in the aqueous phase. The outcomes displayed in the tables above demonstrated that the ligand had the most elevated rate extraction at a pH of 9 for L1H with 92.4% Ni and 87.8% Cu and for L2H with 90.6% Ni and 86.5% Cu atoms extracted from the aqueous into the natural organic stage. Strangely, the ligand additionally recorded extremely decent rate extraction esteems at an acidic pH of 5. This makes the ligand reasonable for extraction in both acidic and basic media.
In this paper, we reported the synthesis of two Schiff base ligands, L1Hand L2H. L1H ligand was formed from the reflux of HNA and AMP while L2H was derived from the reaction of HNA and 2-amino-4,6-dihydroxypyrimidine.The synthesized ligands were characterised using analytical and spectroscopic methods, subsequently evaluated for environmental mob up potentials. The metal ion extraction process was carried out, and obtained result showed a good extractive ability between the ligand and the metal salts (Ni and Cu) in both basic and acidic media with the best at a pH of 9. The electronic data indicated that the Schiff bases assumed no geometry. The IR spectra data for the compounds confirmed the formation of the ligands.
References
- Bhattacharya A, VC Purohit, F Rinaldi (2003) Environmentally friendly solvent-free processes: novel dual catalyst system in Henry reaction, Organic Process Research and Development 7(3): 254-258.
- Qureshi Shabnam, Richards Brian K, McBride, Murray B, Baveye Philippe, et al. (2019) Temperature and Microbial Activity Effects on Trace Element Leaching from Metalliferous Peats. Journal of Environment Quality 32(6): 2067-2075.
- Harvey PJ, Handley HK, Taylor (2015) Identification of the sources of metal (lead) contamination in drinking waters in north-eastern Tasmania using lead isotopic compositions". Environmental Science and Pollution Research 22(16): 12276-12288.
- Howell N, Lavers J, Paterson D, Garrett R, Banati R (2012) Trace metal distribution in feathers from migratory, pelagic birds. Australian Nuclear Science and Technology Organisation.
- Finch LE, Hillyer MM, Leopold MC (2015) Quantitative Analysis of Heavy Metals in Children's Toys and Jewelry: A Multi-Instrument Multitechnique Exercise in Analytical Chemistry and Public Health. Journal of Chemical Education 92(5): 849-854.
- Landis WG Sofield RM, Yu MH (2000) Introduction to Environmental Toxicology: Molecular Substructures to Ecological Landscapes.
- Festus Chioma, Anthony C Ekennia, Aderoju A Osowole, Sunday N Okafor, Collins U Ibeji, et al. (2018) Synthesis, characterization, in-vitro antimicrobial properties, molecular docking and DFT studies of 3-{(E)-[(4,6-dimethylpyrimidin-2-yl)imino]methyl}naphthalen-2-ol and Heteroleptic Mn(II), Co(II), Ni(II) and Zn(II) complexes. Open Chem 16(1): 184-200.
- Li S, S Chen, S Lei, H Ma, R Yu, D Liu (2000) Investigation on some Schiff bases as HCl corrosion inhibitors for copper, Corrosion Science 41(7): 1273-1287.
- Cimerman, S Miljanić, N Galić (2000) Schiff bases derived from aminopyridines as spectrofluorimetric analytical reagents, Croatica Chemica Acta 73(1): 81-95.
- Srivastava S, Goyal P (2010) Decontamination of Toxic Metals from Wastewater. Springer-Verla.

 Short Communication
Short Communication