Abstract
Studies carried out on leguminous plants of the genus Pueraria showed that some species of this genus are endowed with oestrogenic properties and are able to relieve some menopausal symptoms. The aim of thiswork was to assess the potential health benefit of Pueraria phaseoloides in ovariectomized rats, an experimental model of menopause. Ovariectomized Wistar rats were treated for 3-days with aqueous (AE) and dichloromethane/methanol (DCM/MeOH) extracts of Pueraria phaseoloides, following the 14-days interval necessary for functionally perceptible oestrogen decline. Oestrogen-like effects were assessed on primary oestrogen target organs, while anxiolytic properties were evaluated with help of the elevated plus-maze (EPM) and the open field (OF) tests. Then, the effects of extracts on hot flushes were evaluated using data loggers. The DCM/MeOH extract (dose 500 mg/kg) significantly increased the uterine epithelial thickness (p < 0.05). Both AE and DCM/MeOH extracts induced the differentiation of the acini, and led to an increase in eosinophilic secretary granules in mammary gland sat all doses tested. Pueraria phaseoloides induced an increase in the percentages of time spent and number of entries in the open arms of the EPM, in the time spent at the centre of the arena in the OF test. The most pronounced anxiolytic effects were induced by AE at the doses 150 and 300 mg/kg.
Instead, the DCM/MeOH extract had the strongest oestrogen-like effects and reduced significantly the total number (p < 0.05, p < 0.01), the frequency of hot flushes (at all the doses tested), and their average duration at 300 mg/kg (p < 0.01). Our results suggest that Pueraria phaseoloides has oestrogenic properties and can correct various physiological alterations related to oestrogen depletion in ovariectomized rats, possibly through oestrogenic pathway.
Keywords: Puerariapha seoloides; Ovariectomy; Phytoestrogen; Anxiolytic Effects; Hot Flushes; Behavioural Tests
Abbreviations: AE: Aqueous Extract; BW: Body Weight; DCM/MeOH: Dichloromethane/ Methanol; DZP: Diazepam; E2V: Oestradiol valerate; EPM: Elevated plusmaze; ERs: Oestrogen Receptors; ERα: Oestrogen receptors α; ERβ: Oestrogen Receptors β; GABA: Gamma Aminobutyric Acid; i.p.: Intraperitoneal; N. Griffoniana: Newtonia Griffoniana; OF: Open Field; OVX: Ovariectomized; P. phaseoloides: Pueraria Phaseoloides; SEM: Standard Error of the Mean; SHAM: Sham Operated
Introduction
Menopause is the permanent stop of menstruations due to loss of follicular activity in ovaries [1]. It occurs on average at age of 50 ± 10 years [2] and is recognized after a 12 consecutive months period of amenorrhea, without obvious physiological or pathological causes. Menopause is a stage of biological development of women that implies physiological, psychological, and social changes. During this period of their lives, women are vulnerable to cognitive and physical impairment as well as psychiatric illnesses as anxiety and depression. Symptoms associated with oestrogen deprivation at menopause include loss of libido, sleep disorders, vaginal dryness, depression, irritability, anxiety and vasomotor instability with hot flushes and night sweating [3,4]. These signs may be so severe that they require therapeutic intervention [5]. For instance, vasomotor instabilities affect women’s quality of life and by this, their economic yield. With life expectancy increasing trends, women live nearly one third of their life with the nuisances related to menopause, thus, menopause and its consequences are major medical concerns. Hormone Replacement Therapies are available, but are associated with high risk of cardiovascular diseases and breast cancer [6]. Selective modulators of oestrogen receptors like tamoxifene and raloxifene may improve menopause signs without the later risks, but in turn, they may increase the risk for developing other solid cancers [7] and stroke [8]. Consequently, new therapies that can relieve menopausal symptoms with little or no side effects are needed.
The use of medicinal plants has been increasing among menopausal and postmenopausal women worldwide [9,10]. In the same light, many researchers are exploring the potential of phytoestrogens, plant-derived compounds with estrogenic properties, for menopausal disorders’ management. These molecules are structural analogues of mammalian oestrogen 17β-estradiol; thus, they can bind both oestrogen receptors α (ERα) and ERβ to mimic oestrogenic actions in mammals [11]. From this basis, we focused our attention on Pueraria phaseoloides (Roxb.) Benth. (Fabaceae), leguminous specie that grows all over sub-Saharan Africa, Southern Asia, and South and Central America. This plant is used in African traditional medicine to treat ocular diseases and blennorrhoea [12]. Plants of Pueraria genus are endowed with oestrogenic properties and are effective in the treatment of some menopausal symptoms [13-15]. The following phytoestrogens were isolated from Pueraria species: miroestrol, puerarin, deoxymireostrol, kwakhurin, among other compounds of coumestrol class. A study carried out by Cordial and collaborators (2006) on the potential oestrogenic bioactivity of tropical kudzu (Pueraria phaseoloides Roxb. Benth) crude extracts (ethanolic extract) has shown that this plant extract (at doses of 1050, 787.5, 525 mg/kg/day) exhibited weak estrogenic effects in immature ovariectomized female Sprague-dawley rats [15].
In the present study, we assessed the oestrogen-like effects of P. Phaseoloides extracts (aqueous and dichloromethane/ methanol extracts) on primary oestrogen target organs, as well as their anxiolytic activities and ability to improve hot flushes in ovariectomized Wistar rats.
Materials and Methods
Animals
Young female Wistar rats (10-14 weeks, 130-170 g) were used for this study. The animals were housed in a controlled environment: 25°C; humidity 50-80%; 12:12-hlight–dark cycle. They had access to a standard soy-free rat diet and tap water. The experimental procedures were reviewed and approved by the Institutional Ethical Committee of the Cameroonian Ministry of Scientific Research and Innovation, which adopted European Union guidelines on animal use in scientific research (CEE Council 86/ 609; Reg.no.FWA-IRD0001954). The chemicals used in the present study are shown in (Table 1).
Plant Material and Extracts
Pueraria phaseoloides (Fabaceae) whole plant was collected in Mbalngong, (Centre region, Cameroon) and identified at the National Herbarium of Cameroon (HNC) (voucher number 33724/ HNC). After drying and grinding the plant, two methods of extraction were used. For the aqueous extraction, 400g of the powder obtained was macerated in 7L of water for 72h at room temperature. The supernatant was collected and filtered with what man No.4 filter paper. The filtrate was lyophilized, and 24.90g of dried extract was obtained (yield: 6.23%). For the dichloromethane-methanol (DCM/ MeOH) extraction, 500 g of the powder was macerated in 2L of a mixture of dichloromethane and methanol (1:1) for 72h at room temperature. The combined solutions were evaporated under reduced pressure using a rotary evaporator, resulting in 19.43 g of extract (yield: 4.54%). For the two extracts, the doses tested were 150, 300 and 500 mg/kg BW.
Uterotrophic Assay
The female Wistar rats used (N = 54), were bilaterally ovariectomized (N = 48) using the dorsal approach [16] orsham operated (N = 6), under anaesthesia (10 mg/kg diazepam and 50 mg/kg ketamine,i.p., respectively). Fourteen days after surgery (at marked oestrogen decline) [16], animals were randomly distributed into 9 groups (N = 6per group). Sham and untreated ovariectomized control (OVX) groups received distilled water only. A third group received oestradiol valerate (1mg/kg, i.p.) (positive control, E2V). The remaining six groups (test groups) received either the aqueous extract (AE) or the dichloromethane/methanol extract (DCM/MeOH) of P. phaseoloides at doses of 150, 300 and 500 mg/kg. Treatments were doneby gavage in a volume of 10 mL/kg for 3-days. Twenty-four hours after the last administration, animals were sacrificed under deep anaesthesia. Uterine wet weights and total protein levels were determined. The uterus, the vagina and the mammary gland were fixed in 10% formalin for histopathological analysis.
Histopathological Analysis
Changes in the morphology of the mammary glands, as well as the uterine and vaginal epithelial thickness, were assessed on 5-μm sections of paraffin-embedded tissues, following hematoxylin– eosin staining. As in our previous studies [17], a Zeiss (Hallbermoos, Germany) computerized bright field microscopy system was used. It included a microscope (Axioskop 40), and a computer equipped with MRGrab1.0 and Axio Vision 3.1 software (Zeiss).
Behavioural Tests and Hot Flushes
In this part of the study, 10 groups of female rats were used (N = 6 per group): 9 groups were bilaterally ovariectomized while one was sham operated group. Fourteen days after, they were acclimatized in the experimental room for 3-days. Then, the animals were treated with distilled water (sham and OVX groups), with diazepam (DZP, i.p.) oroestradiol valerate (1 mg/kg, E2V) for positive controls [18,19], and with the previously mentioned doses of P. Phaseoloides AE and DCM/MeOH extracts (150, 300 and 500 mg/kg) for the test groups. For each paradigm, a new set of animals were used to avoid the influence of repeated experience on anxiolytic activities of drugs. During the test period, the behaviour of the animal was recorded using a computerized video recording system. The maze or arena was cleaned with 70° ethanol solution between two tests. The experiment was carried out in a calm room and under natural daylight, between 8 am and 4 pm.
Elevated Plus Maze Test: The Elevated plus-maze (EPM) paradigm is one of the most used tests for the study of the spontaneous behaviour of animals. It is a well-accepted and validated behavioural model for detecting the effectiveness of anxiolytic drugs [20]. The maze consisted of two opposite open arms (50 cm×15 cm), crossed with two enclosed arms of the same dimensions with walls 50 cm high. The arms were connected by a central platform (15 cm×15 cm), forming a plus sign. The maze was elevated 71 cm above the floor in a dimly lit room. One hour after administration of the substances, rats were individually placed at the centre of the maze, their heads facing an open arm and allowed to explore the maze for 5 min [18,19]. The following parameters were scored from video recordings offline: the time spent and number of entries in each type of arms, the number of rearing episodes, the number of head dipping and the number of grooming episodes [21]. For each animal, the percentages of time spent and number of entries in open and closed arms were calculated.
Open Field Test: The open field (OF) arena was a wooden square box 60 cm x 60 cm x 40 cm. The floor was divided into16 smaller squares of equal dimensions (15 cm x 15 cm). Each rat was placed at the centre of the arena and allowed to explore it for 5 min. To evaluate the effects of the plant extracts on both exploratory activity and anxiety, the rats were observed for 5 min [18,19]. The parameters scored were: the time spent at the centre of the arena, the number of line crossing, the number of rearing episodes, and the number of grooming episodes.
Effects on Hot Flushes
The rat model for hot flushes introduced by Zingueet al [22] was used to assess the ability of P. phaseoloides extracts to alleviate hot flushes. Recordings of core body temperature with data loggers started 6-h before the beginning of the treatment and ended at day three of the treatment. The recording sessions lasted 72-h and were performed as previously described [16,23], in intervals of 2-min. Only the DCM/MeOH extract, which showed the most promising estrogenic effects in the uterotrophic assay (see Results section), was tested on hot flushes. Female Wistar rats (N = 30) received a single intramuscular dose of penicillin (10 mg/kg) and diclofenac (3 mg/kg) 24-h before ovariectomy. Then, 25 rats were bilaterally ovariectomized using the dorsal approach [16] under Diazepam and ketamine anaesthesia, while the remaining 5 were sham operated. Data loggers were also implanted in the abdominal cavity of the animals during these surgeries. After 14 days, animals were randomly distributed into 5 groups (N = 5per group) and treated for 3 consecutive days with: (i) distilled water (OVX and Sham groups); (ii) oestradiol valerate (1mg/kg) (E2V); and (iii) DCM/MeOH extract at doses 150, 300 and 500 mg/kg (per os). After animal sacrifice, core body temperature data were retrieved from loggers and analyzed using the Smart Button Software (ACR System) for markable changes in core temperature. Hot flushes-like events were episodes of core temperature beyond 38°C [22-23]. The total number, the average duration, and the frequency of hot flushes were determined.
Statistical Analysis
The statistical significance of differences between OVX group or Sham group and test groups were determined using one-way ANOVA followed by Dunnett’s post hoc test, and the significance of the difference between OVX group and Sham group was determined using the unpaired t-test (Graph Pad Prism, version 5.03). Differences with p-value < 0.05 were considered significant. Data were expressed as mean ± standard error of the mean (SEM).
Results
Effects of AE and DCM/ MeOH Extract Son Oestrogen Primary Targets
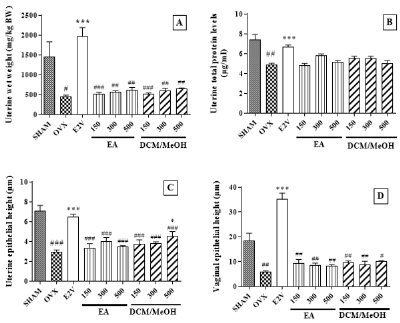
Uterine Wet Weight, Total Protein Levels And Epithelium: As shown in (Figures 1 A-C), 14-days of oestrogen depletion induced significant decreases in uterine wet weight, epithelial thickness, and total uterine protein, respectively, compared to sham animals. Oestradiol valerate significantly increased all these parameters (p < 0.001). While uterus of animals of OVX group displayed a short cuboidal epithelium, E2V group animal shad tall cuboidal to columnar epithelia containing large cells (Figure 2). Groups treated with aqueous and DCM/MeOH extracts showed a non-statistically significant increase in their uterine wet weight, and total protein levelsat all doses tested (Figures 1A & 1B). Uterine epithelium thickness was also increased at all tested doses, with significant increase (P < 0.05) in the group treated with DCM/MeOH at the dose of 500 mg/kg (Figures 1C & 2).
Figure 1: Effects on estrogen primary targets.
Effects of Pueraria phaseoloides extracts on the uterine wet weight
(A) Uterine total protein levels
(B) Uterine epithelial thickness and
(C) Vaginal epithelial height
(D) SHAM= Sham operated rats treated with the vehicle; OVX = ovariectomized animals treated with the vehicle; E2V = ovariectomized animals treated with oestradiol valerate at 1 mg/kg BW; EA = ovariectomized animals treated with the aqueous extract of Pueraria phaseoloides; DCM/MeOH = ovariectomized animals treated with the dichloromethane/methanol extract of Pueraria phaseloides. ANOVA followed by Dunnett’s post hoc test:*P < 0.05, **P < 0.01, ***P < 0.001 vs. OVX and #P< 0.05, ##P < 0.01, and ###P < 0.001 vs. sham.
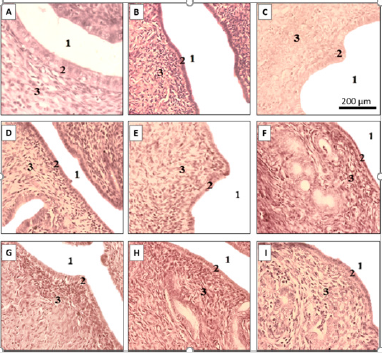
Vaginal Epithelial Thickness: Changes in vaginal epithelial thickness induced by ovariectomy are shown in (Figure 1D), notably a significant decrease in vaginal epithelial thickness (5.68 ± 0.61 μm vs. 18.17 ± 3.22 μm with sham, p< 0.01). A significant increase in vaginal epithelial thickness was observed in E2V group (p < 0.001compared to OVX group). However, increases in vaginal epithelial height were not statistically significant in groups treated with extracts, unlike E2V (Figure 1D). Vaginal epithelia of negative control animals consisted simply of a stratum germinativum, made up of a few layers of flattened cells (Figure 3A & 3B). The treatment with E2V induced the stratification and cornification of the vaginal epithelium of treated animals (Figure 3C). No similar changes were observed in vaginal epithelium of animals treated with the extracts (Figures 3D-I).
Figure 2: Effects on uterine epithelium.
Uterine epithelia of representative cases of sham operated animals and
A. Ovariectomized animals receiving the vehicle
B. Treated with oestradiol valerate
C. Treated with doses 150, 300, and 500 mg/kg of Pueraria phaseoloides aqueous extract
D. (D-F) or Dichloromethane-methanol extract
E. (G-I) 1: Uterine lumen, 2: Endometrium, 3: Stroma.
Figure 3: Effects on the vaginal epithelium.
Vaginal epithelia of representative cases of sham operated animals and
A. Ovariectomized animals receiving the vehicle
B. Treated with oestradiol valerate
C. Treated with doses 150, 300, and 500 mg/kg of Pueraria phaseoloides aqueous extract
D. (D-F) or dichloromethane-methanol extract
E. (G-I) 1: lumen, 2: stratum corneum, 3: epithelium, 4: Stroma.
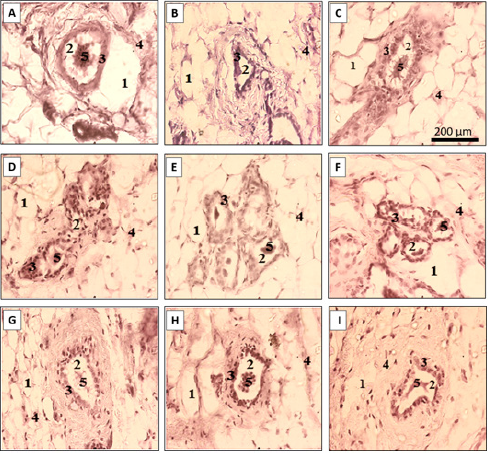
Mammary Gland: (Figures 4 A-4I) show the mammary glands respectively, of sham operated animals (Figure 4A), and ovariectomized animals receiving the vehicle (Figure 4B), treated with oestradiol valerate (Figure 4C), or treated with doses 150, 300, and 500 mg/kg of P. phaseoloides aqueous extract (Figures 4D-4F) or dichloromethane-methanol extract (Figures 4G-4I). Ovariectomy induced the atrophy of mammary glands, with areduced number of parenchyma gland, reduced eosinophil secretion, reduced ductal and alveolar components, as well as modest alveolar development, and prominent adipose tissue (Figure 4B). As in the case E2V group (Figure 4C), there was an increase in eosinophil secretion in lumen of alveoli of animals treatment with P.Phaseoloides extracts, at all tested doses (Figure 4D-4I).
Figure 4: Effects on the mammary gland.
Mammary glands of representative cases of sham operated animals and
A. Ovariectomized animals receiving the vehicle
B. Treated with oestradiol valerate or
C. Treated with doses 150, 300, and 500 mg/kg of Pueraria phaseoloides aqueous extract or
D. (D-F) Dichloromethane-methanol extract
E. (G-I) 1: adipose tissue, 2: lumen of alveoli, 3: alveoli epithelium, 4: gland parenchyma, 5: eosinophil secretion.
Effect of AE and DCM/ MeOH Extracts On Mood And Cognition
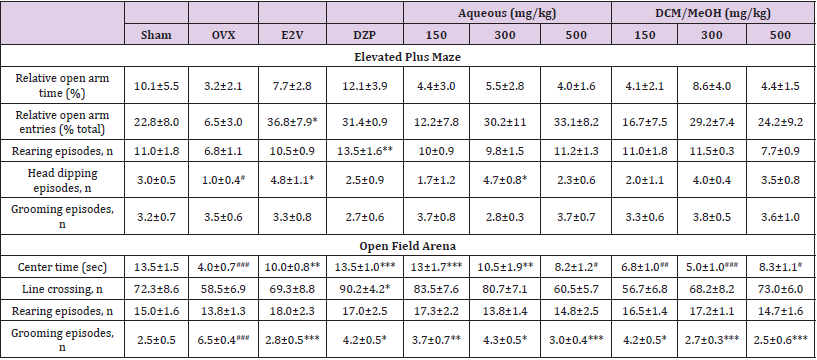
Results of EPM and OF tests are shown in (Table 2).
Elevated Plus Maze: Ovariectomy resulted in the reduction of the percentages of time spent and number of entries in the open arms of the EPM and increased these parameters in the closed arms (Table 2). As expected, these changes were corrected by diazepam and oestradiol valerate (P < 0.05) treatments (positive control groups), as treated ovariectomized animals entered more frequently and spent more time in open arms (Table 2). P. Phaseoloides extracts also induced increases in the percentages of time spent and number of entries in the open arms, but with a high variability, explaining the absence of statistical significance of these changes compared to OVX group (Table 2). Ovariectomy also induced decreases in the number of head dipping episodes (p < 0.05 vs. sham group) and rearing, and increased the number of grooming episodes (Table 2). Oestradiol valerate, diazepam (p < 0.01), and P. Phaseoloides extracts increased the number of rearing (Table 2). The number of head dipping episodes was significantly increased by oestradiol valerate and the dose 300 mg/kg of P. phaseoloides aqueous extract (p<0.05), while non-significant increases were observed in the other test groups (Table 2).
Open Field Test: Ovariectomy induced a significant decrease in the time spent at the centre of the OF arena (p < 0.001vs. sham group), an increase in the number of grooming episodes (p < 0.001), as well as non-significant decreases in line crossing and rearing episodes. The above parameters were highly improved by oestradiol valerate and diazepam (Table 2). Aqueous extract induced a significant increase in the time spent at the centre of the OF arena at doses 150 mg/kg (p < 0.001 vs. OVX group) and 300 mg/ kg (p< 0.01 vs. OVX group) (Table 2); and non-significant increases in the number of line crossings at all doses (Table 2). Aqueous and DCM/MeOH extracts induced significant reductions in the number of grooming episodes at all doses tested (p<0.05; p<0.01; p<0.001) compared with OVX group (Table 2).
Table 2: Effects of P. phaseoloides extracts on some behavioural parameters using the EPM and OF tests.
Table presenting the results from the elevated plus maze (EPM) and open field (OF) tests. Data are expressed as mean ± SEM, n=6 per group. Sham: sham operated animals treated with vehicle, OVX: ovariectomized animals treated with the vehicle, E2V: OVX animals treated with oestradiol valerate at 1 mg/kg BW, DZP: OVX animals treated with diazepam 1 mg/kg BW, Aqueous and DCM/MeOH represents the various treatments by P. phaseoloides extracts. ANOVA followed by Dunnett’s post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001 vs. OVX; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. SHAM.
Effect of DCM/ MeOH Extracts On Hot Flushes
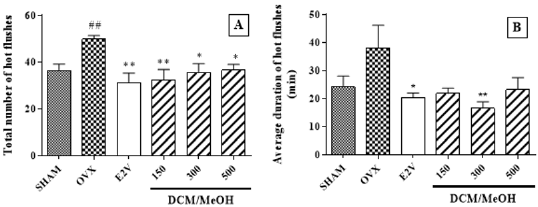
Total Number And Average Duration: Ovariectomy resulted in an increased number (P < 0.01 vs. SHAM group) (Figure 5A) and duration of hot flushes (Figure 5B). Asoestradiol valerate (P < 0.01vs. OVX group), all doses of P. Phaseoloides DCM/MeOH extract decreased the number of hot flushes (P < 0.01; P < 0.05 vs. OVX group) (Figure 5A). In addition, oestradiol valerate (P < 0.05) and the dose 300 mg/kg of P. phaseoloides DCM/MeOH extract decreased the duration of hot flushes episodes(p < 0.01) (Figure 5B).
Figure 5: Effects on hot flushes.
Effects of a Pueraria phaseoloides treatment on total number
(A) and average duration
(B) of hot flushes (3-days treatment) in ovariectomized animals. SHAM= Sham operated rats treated with the vehicle; OVX = ovariectomized animals treated with the vehicle; E2V = ovariectomized animals treated with oestradiol valerate at 1 mg/ kg BW; DCM/MeOH = ovariectomized animals treated with the dichloromethane/methanol extract of Pueraria phaseloides. ANOVA followed by Dunnett’s post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001 vs. OVX.
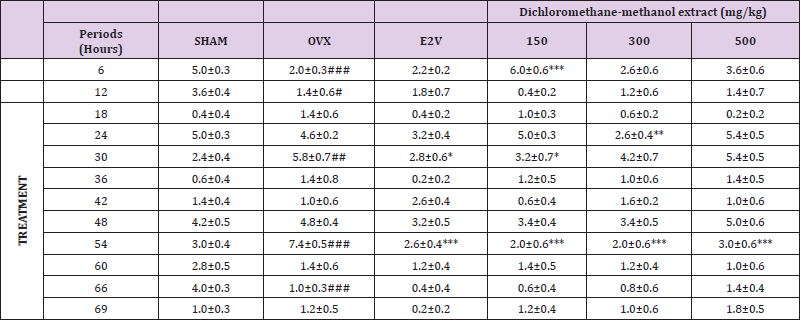
Frequency: The effects of P. phaseoloides treatment on the average frequency of hot flushes are shown in (Table 3). Fourteen days after ovariectomy, the frequency of hot flushes significantly increased in OVX animals at intervals of 30 and 54h (p< 0.05 and p< 0.001 respectively) as compared to normal animals (Sham). Treatment with E2V significantly (p< 0.05; p <0.01) reduced the frequency of hot flushes at 30 and 54 intervals. The most striking reduction of hot flushes’ frequency was induced by P. phaseloides DCM/MeOH extract at dose 300 mg/kg (Table 3).
Table 3: Effects of P. phaseoloides treatment on the average frequency of hot flushes (6-h periods).
Ovariectomized animals receiving the vehicle (OVX) or oestradiol valerate (E2V). ANOVA followed by Dunnett’s post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001 vs. OVX; #P< 0.05, ##P < 0.01, and ###P < 0.001 vs. SHAM.
Discussion
In this study, ovariectomy induced various signs of oestrogen depletion. Notably, reductions in uterine relative weight, epithelial thickness, and total protein level were observed. As expected, treatment with oestradiol valerate improved these signs [24- 25]. At all doses tested, treatments with the aqueous and DCM/ MeOH extracts of P. Phaseoloides improved the relative uterine wet weight and the total proteins level, but with a high inter-individual variability. Similarly, only the test group receiving the dose 500 mg/kg ofDCM/MeOH extract displayed a statistically significant improvement in uterine epithelial thickness, although all the doses of the extract seemed to have improved this parameter. These observations indicate oestrogen-like activities [19,22]. According to some authors, substances with estrogenic properties act on the uterus by water imbibitions and/or cell proliferation, inducing the increase of its weight and/or the high of its epithelium [16,26]. The extracts of P. phaseoloides would thus contain secondary metabolites capable either to increase the vascular micro permeability of the uterus, generating the infiltration of water after trans activation of ERs, or to lead to the synthesis of specific proteins by initiating a cascade of genomic reactions after binding to the ERs of the uterus [27].
Ovariectomy also resulted in a significant decrease invaginal epithelial thickness, which was corrected by oestradiol valerate, partly through stimulation of proliferation, stratification, and cornification of the vaginal epithelial cells [28-29]. P. Phaseoloides extracts induced increases in vaginal epithelial thickness at all the tested doses, but with a high inter-individual variability. These observations are in agreement with several studies, which showed that oestrogens and substances with oestrogenic properties promote the proliferation and differentiation of vaginal epithelium cells, to give stratification and cornification of vagina [16, 22, 30, 31]. These results suggest that the plant has a weak vaginotrophic activity. As previously reported by other investigators [32-33], in this study, ovariectomy resulted in a reduction of the lumen of alveoli, an absence of eosinophil secretions, an atrophied and an undifferentiated epithelium. Treatments with P. Phaseoloides aqueous and DCM/MeOH extracts increased the diameter and the lumen of alveoli, and induced an abundant eosinophil secretion in alveoli lumen at all doses. These results corroborate the observations made by [16,34,35], according to which the regression of the mammary gland induced by ovariectomy is reversed by the administration of oestrogen-like substances. Given the fact that this parameter is an indicator of oestrogenicity at the level of the mammary gland [16,19], it appears that P. Phaseoloides has tissuespecific oestrogen-like properties.
In this study, cognitive and mood alterations were assessed using the elevated plus maze test and open field ethological test. The untreated ovariectomized animals spent less time and entered rarely in the open arms of the elevated plus maze (unsafe area) and were less active than those of sham group, with less rearing, head dipping, and more grooming episodes. All these effects reflect the anxiogenic responses of ovariectomy in the EPM [19]. Indeed, the hormonal changes occurring in the brain following ovariectomy affect negatively the behaviour of rats, by increasing the quantity of indicators of anxious disorders [3]. ¶As oestradiol valerate and diazepam, P. Phaseoloides aqueous and DCM/MeOH extracts increased the percentages of time spent and number of entries in the open arms, and increased the number of rearing and head dipping in both closed and open arms of the maze. According to Lister (1990) [36] and Oviedo et al. [37], the increase in the activity in the open arms directly reflects a reduction of anxiety and the reduction in the activity in the closed arms shows a decrease of stress. Indeed, some investigations indicate that the administration of oestrogens or oestrogenic compounds in ovariectomized rats produces anxiolytic-like effects, possibly associated with the reactivation of brain neurotransmission [38]. These observations suggest that P. Phaseoloides may contain secondary metabolites endowed with anxiolytic properties as suggested by Djiogue et al. [39] for a medicinal plant (N. Griffoniana).
In the same light, the open field test also revealed signs of mood alterations and cognitive impairment in ovariectomized animals [40-41], mainly by reductions in the number of line crosses, in the number of rearing episodes, in the time spent in the centre of the arena (unsafe area), as well as increases in the number of grooming episodes. Line crossing and rearing episodes’ numbers were increased by both the aqueous and DCM/MeOH extracts of P. phaseoloides, but with a high inter-individual variability. On the other hand, P. Phaseoloides extracts induced significant decreases in the number of grooming episodes at all the doses tested. The time spent in the centre of the arena was increased significantly by aqueous extract doses 150 and 300 mg/kg. These results also suggest that P. phaseoloides may contain compounds with anxiolytic properties. Such anxiolytic properties could result from the action of these compounds on the action sites of benzodiazepines in the gamma aminobutyric acid (GABA) receptors complex [42]; on the monoaminergic neurotransmission, affecting the production and release of nor epinephrine, dopamine, and serotonin in the brain [43]; or may be from their effects on the density of oestrogen receptor α and β in the hippocampus, and on the activity of glutamate receptors [44].
In this way, these data support the hypothesis that P. Phaseoloides could be a possible therapeutic alternative in the natural management of anxiety disorders in menopausal women. Menopause is associated with physiological and psychological changes partly inducible experimentally by ovariectomy in rats, such as vasomotor symptoms, hot flushes, palpitation, vaginal dryness, osteoporosis, irritability, anxiety and other mood alterations, and cognitive signs [25]. In this study, ovariectomy induced significant increases in the number, frequency, and duration of hot flushes episodes, as expected [45], which were corrected by oestradiol valerate. These results corroborate the assertion according to which hot flushes occur in response to ovariectomy in female mammals and are effectively treated by oestrogen replacement [46] or by phytoestrogens (geniste in) intake [47-48]. Indeed, it is believed that oestrogen increases the size of the thermo neutral zone and raises the sweating threshold thus, reducing the frequency of hot flushes [45,49]. P. Phaseoloides DCM/MeOH extract induced significant reductions in the total number and frequency of hot flushes at all the doses tested. Only the dose 300 mg/kg reduced significantly the average duration of hot flushes. These effects further support the hypothesis of the presence of compounds endowed with oestrogen-like activity in P. phaseoloides, which would have reversed the thermoregulatory dysfunction related to the endogenous estrogens decline caused by ovariectomy [24].
Conclusions
The aim of this study was to assess the oestrogen-like effects of aqueous and dichloromethane-methanol extracts of P. Phaseoloides on oestrogen primary targets, as well as their anxiolytic activities and potential beneficial effects against hot flushes in ovariectomized Wistar rats. P. Phaseoloides extracts displayed oestrogen-like activities on target organs and anxiolytic properties in ovariectomized rats. In addition, the DCM/MeOH extract reduced significantly the total number, average duration, and frequency of hot flushes. Altogether, these observations suggest that P. Phaseoloides may contain compounds with oestrogenlike properties, whose bioactivity was strong enough to correct oestrogen depletion-related alterations at tissue level in this study.
Declarations
Ethics Approval
All experiments were carried out in accordance with the Cameroon National Ethic Committee for animal experiments, which adopted all procedures recommended by the European Union on the protection of animals used for scientific purposes (CEE Council 86/ 609; Reg. no. FWA-IRD 0001954).
Consent for Publication
Not applicable
Availability of Data and Material
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interest
All the authors declare no conflicts of interest within this article.
Funding
We received no funding support for the present study.
Authors Contributions
DS conceived the study, supervised the data collection and analysis as well as the manuscript preparation. MTF performed the experiments and data collection, participated to the first draft manuscript writing. DTRN participated to the data collection and experimental part. ZGF participated to the data collection and experimental part and data analysis. ACF participated to the data collection and experimental part. SEPF participated to the study conception and manuscript writing. KWGJM participated to the manuscript reading and data analysis. ND participated to the study conception and manuscript proof reading.
Acknowledgments
The authors are thankful to the members of the Laboratory of Animal Physiology of the University of Yaoundé 1 for their technical assistance.
References
- Nair PA (2014) Dermatosis associated with menopause. J Midlife Health 5(4): 168-175.
- Bahiyah A, Burhanuddin M, Badrul AI, Madihah Z, Najih FMN, et al. (2017) Prevalence of menopausal symptoms, its effect on quality of life among Malaysian women and their treatment seeking behaviour. Med J Malaysia 72(2): 94-99.
- Rodríguez Landa JF, PugaOlguín A, GermánPonciano LJ, García Ríos RI, Soria Fregozo C, et al. (2015) Anxiety in Natural and Surgical Menopause -Physiologic and Therapeutic Bases. A Fresh Look at Anxiety Disorders 9: 174-190.
- Pinkerton JV, Stovall DW, Kightlinger RS (2009) Advances in the treatment of menopausal symptoms. Women's Health 5(4): 361-384.
- Tong IL (2013) Nonpharmacological treatment of postmenopausal symptoms. The Obstetrician & Gynaecologist 15: 19-25.
- Goodman NF, Cobin RH, Ginzburg SB, Katz IA, Woode DE, et al. (2011) American association of clinical endocrinologist guidelines for medical clinical practices for the diagnosis and treatment of menopause. Endocrine Practice 17.
- Traboulsi T, Ezzy ME, Gleason JL, Mader S (2017) Antiestrogens: structure-activity relationships and use in breast cancer treatment. J MolEndocrinol 58: 15-31.
- Gambacciani M (2013) Selective estrogen modulators in menopause. Minerva Ginecol 65(6): 621-30.
- Pitkin J (2012) Alternative and complementary therapies for menopause. Menopause Int 18: 20-27.
- Ekor M (2014) The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in Pharmacology 4(177): 1-10.
- Schilling T, Ebert R, Raaijmakers N, Schütze N, Jakob F, et al. (2014) Effects of phytoestrogens and other plant-derived compounds on mesenchymal stem cells, bone maintenance and regeneration. J Steroid BiochemMolBiol 139: 252-261.
- Dweck CA (2002) The Pueraria family with special interest in Puerariamirifica. Personal Care Mag 3(1): 7-10.
- Kamiya T, Akira T, Yayoi K, Yuki M, Mayu SK, et al. (2013) Evaluation of the Estrogenic Activity of Pueraria (Kudzu) Flower Extract and Its Major Isoflavones Using ER-Binding and Uterotrophic Bioassays. Pharmacol Pharm 4: 255-260.
- Kakehashi A, Midori Y, Yoshiyuki T, Naomi I, Takahiro O, et al. (2016) Puerariamirifica Exerts Estrogenic Effects in the Mammary Gland and Uterus and Promotes Mammary Carcinogenesis in Donryu Rats. Toxins 8(11): 1-15.
- Cordial RR, Bella MD, Paul MSF, Abigail SG, Rod MMC, et al. (2006) Estrogenic Activity of PuerariaphaseoloidesRoxb. Benth evaluated in ovariectomized rats. Philippine J Sc 135: 39-48.
- Njamen D, Djiogue S, Zingue S, Mvondo MA, Nkeh Chungag NB, et al. (2013) In vivo and in vitro estrogenic activity of extracts from Erythrinapoeppigiana (Fabaceae). J Complement Integr Med 10(1): 1-11.
- (2007) OECD, Organization of Economic Cooperation and Development. Third meeting of the validation management group for the screening and testing of endocrine disrupters (mammalian effects). Joint meeting of the chemicals committee and the working party on chemical, pesticides and biotechnology.
- Zemo GF, Djiogue S, Ketcha WGJM, Seke PFE, Yonkeu TFG, et al. (2017) Fourteen days post-ovariectomyestrogens decline is associated with anxiogenic effects on Wistar rats. J Pharm Pharmacol 5: 869-876.
- Ketcha WGJM, Zemo GF, Djiogue S, Awounfack CF, Njamen D, et al. (2017) Estrogenic and anxiolytic effects of the decoction of stem bark of Khayaanthotheca (Welw.) C.DC (Meliaceae) in ovariectomizedWistar rats. Inter J Phytomed 9: 241-252.
- Walf AA, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behaviour in rodents. Nat Prot 2(2): 322-328.
- Casarrubea M, Roy V, Sorbera F, Magnusson MS, Santangelo A, et al. (2013) Temporal structure of the rat's behaviour in elevated plus maze test. Behav Brain Res 237: 2909.
- Zingue S, Michel T, Tchatchou J, Magne NCB, Winter E, et al. (2016) Estrogenic effects of Ficus umbellate Vahl. (Moraceae) extracts and their ability to alleviate some menopausal symptoms induced by ovariectomy in Wistar rats. J Ethnopharmacol. 179: 332-44.
- Nkeh Chungag BN, Tiya S, Mbafor JT, Ndebia EJ, Rusike S, et al. (2013) Effects of the methanol extract of Erythrinaabyssinica on hot flushes in ovariectomized rats. Afr J Biotechnol 12: 598-601.
- Salleh N, Helmy MM, Fadila KN, Yeong SO (2013) Isoflavone Genistein Induces Fluid Secretion and Morphological Changes in the Uteri of Post-Pubertal Rats. Int J Med Sci 10(6): 665-675.
- Mazo DJ (2013) Reproductive Toxicology: In Vitro Germ Cell Developmental Toxicology, from Science to Social and Industrial Demand. Springer Science & Business 223.
- Takahashi O, Oishi S, Yoneyama M, Ogata A, Kamimura H, et al. (2007) Antiestrogenic effect of paradichlorobenzene in immature mice and rats. Arch Toxicol 81(7): 505-517.
- Ketcha WGJM, Djiogue S, Djoussi NSO, Awounfack CF, Njamen D, et al. (2016) Evaluation of the estrogenic properties of aqueous extracts of benthamii Baker (Euphorbiaceae) and their ability to alleviate some menopausal symptoms induced by Graptophyllumpictum ovariectomy in Wistar rats Tragia (Acanthaceae). International Journal of Phytomedicine 8: 366-378.
- Abdel Aal IH, Abdel HGA, Selim ME (2015) Restoration of Vaginal Epithelial Atrophy in Ovariectomized Rats with Sex Steroid Hormones. MOJ Anatomy & Physiology 1(2): 2-8.
- Montoya TI, Maldonado PA, Acevedo JF, Word RA (2015) Effect of Vaginal or Systemic Estrogen on Dynamics of Collagen Assembly in the Rat Vaginal Wall. BiolReprod 92(2): 43.
- Sachin R, Patil A, Patil MB, Bhatkal DL (2012) Estrogenic activity of various extracts of plumbagozeylanica roots in female rats. Plant Arch 12: 509-513.
- Basha ME, Chang S, Burrows LJ, Lassmann J, Wein AJ, et al. (2013) Effect of estrogen on molecular and functional characteristics of the rodent vaginal muscularis. J Sex Med 10: 1219-1230.
- Lecomte S, Demay F, Ferrière F, Pakdel F (2017) Phytochemicals Targeting Estrogen Receptors: Beneficial Rather Than Adverse Effects? Int J MolSci 18(7): 1381.
- Santos MA, Florencio Silva R, Teixeira CP, Sasso GR, Marinho DS, et al. (2016) Effects of early and late treatment with soy isoflavones in the mammary gland of ovariectomized rats. Climacteric 19(1): 77-84.
- Santell RC, Chang YC, Nair MG, Helferich WG (1997) Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. J NutrEduc 127: 263-269.
- Zingue S, Njamen D, Tchoumtchoua J, Halabalaki M, Simpson E, et al. (2013) Effects of Millettiamacrophylla (Fabaceae) extracts on oestrogen target organs of female Wistar rat. J PharmacolSci 123: 120-131.
- Lister RG (1990) Ethologically based animal models of anxiety disorders. PharmacolTher 46: 32140.
- Oviedo VM, Milded GG, Rincon J, Guerrero MF (2006) Effect of an extract ofAnnonamuricata on central nervous system. Pharmacologyonline 3: 34247.
- Pandaranandaka J, Poonyachoti S, Kalandakanond Thongsong S (2006) Anxiolytic property of estrogen related to the changes of the monoamine levels in various brain regions of ovariectomized rats. Physiology and Behaviour 87: 828-835.
- Djiogue S, Kinyok MJ, Ketcha WGJM, Zemo GF, Seke EP, et al. (2015)Newtonoate as an active principle of Newtoniagriffoniana for anxiolytic activity in Swiss mice. J ComplIntegr Med 12(4): 283-287.
- Seibenhener ML, Wooten MC (2015) Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behaviour in Mice. J Vis Exp (96): 52434.
- Njapdounke KJS, Nkantchoua NG, Moto OFC, Taiwe SG, Sidiki N, et al. (2016) Anxiolytic - like properties of halleaciliata in mice. Afr J Tradit Complement Altern Med 13(4): 1-7.
- Daendee S, Thongsong B, Kalandakanond Thongsong S (2013) Effects of time of oestrogen deprivation on anxiety-like behaviour and GABAA receptor plasticity in ovariectomized rats. Behavioural Brain Research 246: 86-93.
- Pandaranandaka J, Poonyachoti S, Kalandakanond Thongsong S (2009) Differential effects of the exogenous and endogenous estrogen on anxiety as measured by elevated T maze in relation to the serotonergic system. Behavioural Brain Research 198: 142-148.
- Jin M, Jin F, Zhang L, Chen Z, Huang H (2005) Two oestrogen replacement therapies differentially regulate expression of oestrogen receptors alpha and beta in the hippocampus and cortex of ovariectomized rat. Molecular Brain Research 142(2): 107-114.
- Dacks PA, Rance NE (2010) Effects of oestradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology 151(3): 1187-1193.
- Santoro N (2008) Symptoms of menopause: hot flushes. Clin Obstet Gynecol 51: 539-548.
- Crisafulli A, Marini H, Bitto A, Altavilla D, Squadrito G, et al. (2004) Effects of genistein on hot flushes in early postmenopausal women: a randomized, double-blind EPT- and placebo-controlled study. Menopause 11(4): 400-404.
- DAnna R, Cannata ML, Marini H, Atteritano M, Cancellieri F, et al. (2009) Effects of the phytoestrogen genistein on hot flushes, endometrium, and vaginal epithelium in postmenopausal women: a 2-year randomized, double blind, placebo-controlled study. Menopause 16(2): 301-306.]
- Freedman RR, Blacker CM (2002) Estrogen raises the sweating threshold in postmenopausal women with hot flashes. FertilSteril 77: 487-490.

 Research Article
Research Article