Abstract
Neuropsychiatric symptoms are near universal among patients with neurodegenerative disorders, thus almost all patients experiencing at least one of them during the course of their illness. Currently, there is no FDA-approved medication for the treatment of neuropsychiatric symptoms in the course of neurodegenerative disorders with the exception of pimavanserin approved for the treatment of psychosis in the course of Parkinson’s disease. Pimavanserin shows benefit in addressing psychosis in Alzheimer’s disease. Although used “off label”, a large number of antidepressants, mood stabilizers, typical and atypical antipsychotics are prescribed for behavioral disturbances in persons with neurodegenerative disorders. In this review, the terminology, clinical features, demographic and epidemiologic facts, diagnostic criteria and management strategies for selected neuropsychiatric symptoms like depression, psychosis with a special attention to apathy and agitation in the course of Alzheimer’s are discussed. Data on the suspected pathophysiologic mechanisms are also reviewed. Several issues relating to future therapies that may impact patients are briefly mentioned.
Keywords: Neurodegenerative Disorders; Alzheimer’s Disease; Neuropsychiatric Symptoms; Depression; Psychosis; Agitation; Apathy; Antipsychotics
Abbreviations: AChEI: Acetylcholinesterase Inhibitor; AD: Alzheimer’s Disease; ADMET: Alzheimer’s Disease Methylphenidate Trial; AE: Adverse Effect; AES: Apathy Evaluation Scale; APA: The American Psychiatric Association; BPRS: Brief Psychotic Rating Scale; BPSD: Behavioral and Psychological Symptoms of Dementia; CBT: Cognitive Behavioral Therapy; CMAI: Cohen-Mansfield Agitation Inventory; CGI-C: Clinical Global Impression of Change; CGI-S: Clinical Global Impression of Severity of Illness; CGI-I: Clinical Global Impression of Improvement; CSDD: Cornell Scale for Depression in Dementia; DPA: Dopaminergic Agonist; DMAS: Dementia Mood Assessment Scale; NBRS-A: Neurobehavioral Rating Scale-Agitation Subscale; NDD: Neurodegenerative Disorders; NPS : Neuropsychiatric Symptoms; MADRS: Montgomery-Asberg Depression Rating Scale; MAOI: Monoamine Oxidase Inhibitor; MMSE: Mini Mental State Examination; MRI: Magnetic Resonance Imaging; NIMH-dAD: The National Institute of Mental Health Depression of Alzheimer’s Disease; NPI: Neuropsychiatric Inventory; PT: Physical Therapy; RCT: Randomized Clinical Trial; SNRI: Serotonin- Norepinephrine Reuptake Inhibitor; SSRI: Selective Serotonin Reuptake Inhibitor; TCA: Tricyclic Antidepressant; rTMS: Repetitive Transcranial Magnetic Stimulation; UPDRS: Unified Parkinson’s Disease Rating Scale
Introduction
Behavioral and psychological symptoms classified as neuropsychiatric symptoms (NPS) commonly accompany dementia syndromes in the course of neurodegenerative diseases (NDD) [1,2]. Cardinal psychiatric symptoms such as depression, apathy, psychosis or agitation complicate the clinical presentation of Alzheimer’s disease (AD) and Parkinson’s diseases (PD) and some are considered core symptoms of frontotemporal dementia and dementia with Lewy bodies (DLB) [1]. NPS are near universal among patients with neurodegenerative disorders (NDD), thus almost all patients with AD experiencing at least one of them during the course of their illness [3]. NPS are usually assessed by comprehensive psychiatric evaluation but more structured tools like the Neuropsychiatric Inventory (NPI) commonly used in the setting of trials can be of help in clinical practice [2]. The group of common and debilitating NPS include apathy, irritability, agitation, depression, delusions, hallucinations, anxiety, disinhibition, aberrant motor behavior, sleep disturbances and eating abnormalities [2]. NPS can manifest themselves at all types and stages of NDD (especially when a dementia syndrome is present), often cluster, tend to be persistent and are associated with excess morbidity and mortality, contribute to patients’ distress and caregiver burden.
They are NPS are linked to increased healthcare use, healthcare costs, and institutionalization [4]. The neurobiology of NPS is extremely complex. Dysfunction in frontal-subcortical and corticocortical networks was proposed as a model of NPS [5]. In terms of neurochemistry dysfunction in ascending monoaminergic systems involving serotonin, norepinephrine and dopamine, glutamatemediated excitatory neurotoxicity, tau-mediated pathology, inflammation play a role in the occurrence of NPS in NDD (Murai et al.); [6,7]. Despite the significant burden of these symptoms, there are few recommended, evidence-based treatments including pharmaceuticals [8,9]. Pharmacotherapy in the geriatric population in general is challenging due to age-associated changes in pharmacodynamics and pharmacokinetics as well as high rates of medical comorbidities and concomitant medications, which increases risk for polypharmacy, drug-drug interactions, and adverse drug effects [9]. Additionally, there are risks attributed to use of antipsychotics, antidepressants, anxiolytics and /or mood stabilizers in elderly, and specific risks to patients with dementia which makes a decision about treatment complicated both for providers and patients and caregivers [8].
Psychotropics, especially antipsychotic medications, may alleviate certain NPS, but may have severe adverse effects including increased risk of involuntary movements, cerebrovascular events, falls, and death [9]. Effectiveness of psychopharamceuticals in the treatment of NPS is a subject of ongoing debate. AD treatments may have effects on NPS and can affect decisions regarding treatment with psychotropic agents. Cholinesterase inhibitors may reduce the emergence of NPS and have a role in their treatment. These agents may delay initiation of or reduce the need for other drugs such as antipsychotics thus ACHI’s should be initiated, optimized and maintained for the management of both cognitive symptoms as well as NPS [7]. Currently, there is no FDA-approved medication for the treatment of NPS in NDD with the exception of pimavanserin which was recently approved for the treatment of psychosis in the course of PD [10]. Although used “off label”, a large number of antidepressants, mood stabilizers, typical and atypical antipsychotics are prescribed for behavioral disturbances in persons with NDD [8,11]. Parallel to robust efforts in pharmacological trials there is an ongoing need to assess and verify existing, as well as create new behavioral strategies.
Cohen-Mansfield [12] conceptualizes behavioral disorders in the course of NDD as representing unmet personal needs such as pain and any other somatic discomfort, need for social contact, and stimulation to alleviate boredom. Those needs should be recognized and successfully addressed by nonpharmacologic interventions. Therapeutic approaches should be individually tailored to each patient with NPS using behavioral management techniques, caregiver education and support, problem solving and communication skills training, music therapy, aromatherapy and modified cognitive behavioral and interpersonal therapies. With few exceptions initiating pharmacotherapy should occur only after treating or eliminating underlying medical or environmental factors and should be limited to cases where nonpharmacological measures have failed [12]. All patients should be carefully monitored for development of adverse events and side effects during a timelimited treatment course; symptoms often resolve over time regardless of medication use [11]. An ongoing assessment of benefit versus harm should continue throughout the course of treatment with periodic consideration of withdrawing the medication [8,13].
Depression of Alzheimer’s Disease
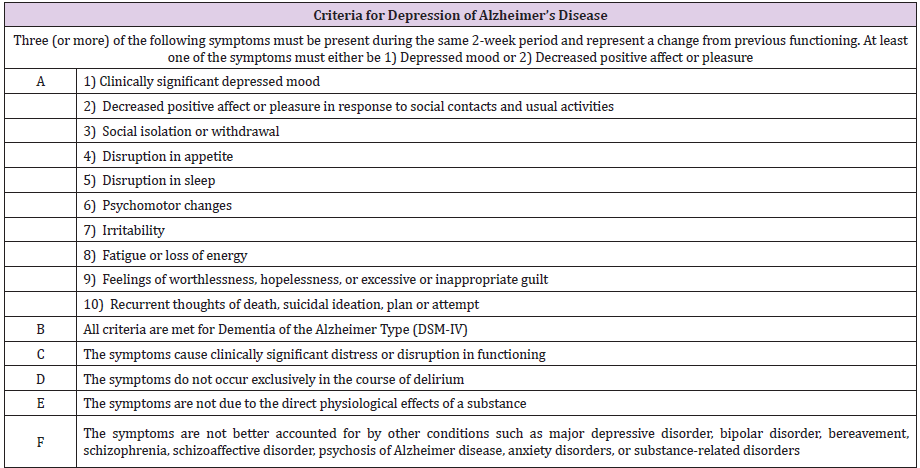
Depression which develops during the course of AD is conceptualized as one of NPS, “depression of AD” [14]. The concept was defined as a depressive syndrome with prominent decreased affect, irritability, agitation, and anxiety, diminished attention and fatigue but less evidence of guilt and suicidality than major depressive episode. Depression of AD is relatively common (affecting up to 50% of persons with AD) and persistent with 50%- 60% of untreated depressed patients with AD remaining depressed at 1-year follow-up [14]. A National Institute of Mental Health Work Group developed a set of diagnostic criteria for depression of AD (NIMH-dAD) (Table 1). These criteria were derived from DSM-IV criteria for major depression, with some distinctions. The number of symptoms required for a diagnosis of depression was decreased from five to three. The duration and frequency of depressive symptoms was also decreased; symptoms need only be present together within the same 2-week period. The decreased ability to think and concentrate was eliminated. The criteria for anhedonia were modified to focus on decreased affect and pleasure associated with social and other activities. Social isolation/withdrawal and irritability were added as new symptoms [15]. So far neither DSM 5 related version nor correction of NIMH-AD had been published.
Table 1. Criteria for depression of Alzheimer’s disease.
Note: Adapted from : Teng E, Ringman JM, Ross LK, Mulnard RA, Dick MB, Bartzokis G, Davies HD, Galasko D, Hewett L, Mungas D, Reed BR, Schneider LS, Segal-Gidan F, Yaffe K, Cummings JL; Alzheimer’s Disease Research Centers of California-Depression in Alzheimer’s Disease Investigators. Diagnosing depression in Alzheimer disease with the national institute of mental health provisional criteria. Am J Geriatr Psychiatry 2008 Jun;16(6):469-77.
Rationale for Interventions and Treatment
Despite the widespread antidepressant use (almost 50% of patient with dementia are on antidepressants) there is mixed evidence regarding the benefits from the use in AD depressed patients. Many trials have been carried out on small numbers of patient and were underpowered to detect differences. Variable trial methods, comorbid conditions and differences in the administered antidepressants further confound findings .Use of antidepressants in AD and other NDD is based on their use in major depressive disorder pending better evidence for or against their use in depression of Alzheimer’s disease. Acetylcholinesterase inhibitors (AChEIs) studies provide some evidence of benefit from their use. Among the NPS in the course of AD most likely to improve appear to be apathy and depression followed by aberrant motor behavior [16]; (Howard et al.). A secondary analysis of patients with severe behavioral disturbances previously treated with donepezil and sertraline, Cummings et al. [17] suggest that donepezil reduces behavioral symptoms, particularly mood disturbances and delusions, in patients with AD. A withdrawal study by Holmes et al. [18] provided additional evidence to support the use of donepezil in the treatment of neuropsychiatric symptoms (including depression) in patients with mild to moderate AD with marked NPS.
Following open-label treatment with donepezil patients randomized to placebo showed a significant worsening of neuropsychiatric symptoms and a worsening of caregiver distress compared with a continued improvement in those who remained on donepezil treatment. Optimization as well as maintenance treatment with AChEIs should be considered as a step in the management of depression of AD. The American Psychiatric Association (APA) recommends a trial of an antidepressant to treat clinically significant, persistent depressed mood in patients with dementia. Selective serotonin uptake inhibitors (SSRIs) are preferred because of their favorable safety profile [19]. Sertraline, citalopram or escitalopram in low doses are the most appropriate first-line agents. Other SSRIs like fluoxetine and paroxetine are not recommended as a first line SSRI’s due to debatable efficacy and unfavorable mostly anticholinergic effects [20]. This dose might be increased weekly, if tolerated, to a maximum of 150 mg of sertraline or 40 mg of citalopram per day with close monitoring of side effects. Although improvement should occur within 4 to 6 weeks at the target dose, a longer period may be required to reach full effect [20].
If patients do not respond to SSRI switching to a different agent or augmenting a treatment with second agent should be considered. Especially for patients who have psychotic symptoms or agitation along with depression, an atypical antipsychotic in a small dose might be considered [21,22]. An anticonvulsant in smaller doses (the best evidence is for carbamazepine) might be considered as additional therapy to an antidepressant if there is moderate or severe agitation [23]. Switching to an antidepressant from a different class (as opposed to augmentation) is recommended in cases of severe side effects induced by initial medication. Preferred second-line agents are selective norepinephrine reuptake inhibitors properties (SNRIs) such as venlafaxine or duloxetine, or antidepressants with a mixed pharmacology (mirtazapine, bupropion). Evidence for benefit from use of non-SSRI antidepressants specifically for depression in AD is lacking. Tricyclic antidepressants are not recommended due to lack of convincing evidence and the occurrence of various side effects including anticholinergic ones. Psychiatric hospitalization should be considered an option in complex severe cases.
For patients with severe, refractory depression, electroconvulsive therapy (ECT) might be considered, especially if there is risk of self-harm or harm to others [20]. There is evidence for ECT as an effective and well-tolerated option for treating depression in people with dementia [24]. The situation of limited effectiveness of pharmaceuticals supports nonpharmacological interventions in depression of AD. Interpersonal psychotherapy (IPT) especially versions modified to address the needs of older adults with mild cognitive deficits (IPT-CI [25]. Cognitive behavioral therapy (CBT) has been adapted for depressed older adults with mild stages of dementia [26]. Methods specifically developed to treat geriatric depression such as home delivered problem adaptation therapy (PATH) or problems solving skills and caregiver training show benefit [27].
Apathy of Alzheimer’s Disease
Extent of the Problem, Pathogenesis and Impact
Apathy, despite being usually clustering with mood symptoms should be rather considered a disorder of motivation. Marin et al. [28] conceptualized apathy as diminished goal-directed activity in the domains of behavior, cognition and emotion. Apathy is considered one of the most common NSP in AD with a 5-year prevalence of 71%. It can cluster with other NPS and is associated with higher costs and burden of care [3]. Apathy can be assessed with a set of criteria by Mulin et al. [29] (Table 2). Diminished motivation in comparison to the patient’s previous level of functioning should be noticed. Diminished motivation should be observable most of the time for a period of at least four weeks and accompanied by additional symptoms like loss or diminished goal-directed behavior or/and cognitive activity or/and emotion. Symptoms should cause clinically significant impairment in personal, social, occupational, or other important areas of functioning and cannot be exclusively explained by physical and/or motor disabilities, diminished level of consciousness or the direct physiological effects of a substance. Growing evidence suggests that apathy in AD is a manifestation of dysfunction of dopaminergic circuits between the basal ganglia, anterior cingulate, and other frontal cortex structures involved in motivation and reward (David et al.).
Table 2. Criteria for apathy in neurodegenerative disorders.
Note: Adapted from: Mulin E, Leone E, Dujardin K, Delliaux M, Leentjens A, Nobili F, Dessi B, Tible O, Agüera-Ortiz L, Osorio RS, Yessavage J, Dachevsky D, Verhey FR, Cruz Jentoft AJ, Blanc O, Llorca PM, Robert PH. Diagnostic criteria for apathy in clinical practice. Int J Geriatr Psychiatry 2011 Feb; 26(2):158-65.
Rationale for Interventions and Treatment
Psychostimulants including methylphenidate and modafinil have been used to treat apathy in AD. Methylphenidate acts by blocking the dopamine transporter and norepinephrine transporter, leading to increased concentrations of dopamine and norepinephrine within the synaptic cleft. Methylphenidate but not modafinil proved to be effective in reducing apathy in AD in a small cross over trial [30] and was further assessed in the larger, multicenter, double-blind controlled trial Alzheimer’s Disease Methylphenidate Trial (ADMET) [31]. In ADMET, methylphenidate (20 mg daily for 6 weeks) was associated with a significant reduction in apathy symptoms. Two study outcomes measures CGI-C and NPI apathy score, showed diminished apathy symptoms with methylphenidate treatment. Adverse events and side effects were modest. The results suggest that methylphenidate treatment may have clinical utility in treating apathy of AD with a potential for improving cognition as well. Besides promising results from dopaminergic agents, AChEIs appear to be successful in improving symptoms of apathy in AD [16]. Symptoms may worsen after discontinuation of AChEIs [18]. Optimization and maintenance treatment with AChEIs should thus be considered as a crucial step in the management of apathy of AD.
Psychosis of Alzheimer’s Disease
Extent of the Problem, Pathogenesis and Overall Impact
Estimates of the incidence of psychosis in AD range widely from 10% to 75% [32]. The common psychotic symptoms reported in the AD patients are delusions and hallucinations followed by misidentification phenomena [1]. Hallucinations are predominantly visual. Auditory phenomena especially of a schizophrenic quality are rare in AD [33]. Delusions in the course of AD are typically paranoid, non-bizarre, and simple. Delusions tend to recur or persist for several years in AD patients, but active and vivid perception and delusions have a tendency to diminish in intensity in the course of cognitive decline, shallow insight and decreasing ability for verbal expression [33]. In a study which compared AD delusional and non-delusional subjects, those with delusions were significantly older, with higher age at onset and cognitive impairment, a more severe stage of dementia, and more depressive symptoms than AD patients with no delusional symptoms.
Disease duration was slightly higher in AD delusional patients than in those without. Delusional patients showed a higher grade of disability in basic and instrumental activities of daily living [34]. Delusions to cluster with hallucinations, agitation/aggression, depression mood, apathy, irritability, aberrant motor activity, sleep disturbances, and eating disorders as assessed by NPI. More severe cognitive impairment and faster rate of cognitive decline are associated with and predictive of hallucinations and delusions in patients with AD. Parkinsonism also is predictive of imminent psychotic symptoms in AD. Psychosis of AD is believed to be the result of dysfunction of frontal lobe circuitry with contributions from neurofibrillary tangles in limbic structures as well as neurochemical abnormalities including the cholinergic deficit and dopaminergic dysfunctions.
Rationale for Interventions and Treatment
AChEIs may reduce or /and postpone the need for the use psychotropics with worse safety profile. In a community dwelling studies (completed by twenty-four patients with AD) donepezil was proved to significantly reduce delusions, hallucinations and agitation in the majority of subjects. Memantine appears to provide modest benefit for the management of AD psychosis and has a favorable safety profile. In a pooled, retrospective analysis of data from three placebo-controlled trials in moderate to severe AD, memantine was linked to significant reduction in psychosis, agitation, and aggression [35]. In more severe psychotic symptoms, which doesn’t respond to AChEIs or/and memantine antipsychotic medications are used. Atypical antipsychotic drugs are more effective than placebo, although adverse effects may limit their overall effectiveness [36]. Antipsychotic medications have been associated with a small but significant decrease in caregiver burden [37]. Olanzapine (up to 10 mg daily) has shown benefit in managing delusions and hallucinations, anxiety and agitation in AD patients [38].
Aripiprazole (5 to 10 mg/day) was efficacious and relatively safe for psychosis associated with AD, significantly improving psychotic symptoms, agitation, and NPS as assessed by NPI, BPRS and CMAI scores [39]. Risperidone (mean doses of 1.5 mg daily) treatment was proved efficacious for psychosis of AD in couple of studies [21]. Quetiapine is also commonly used in off-label indications such as NPS of AD. It is believed to have a lower incidence of serious side effects such as extrapyramidal symptoms and tardive dyskinesia when compared with other antipsychotics. Quetiapine can be used in a wide range of doses. Sedating properties are of some use in certain clinical situations in NDD [40]. Results may vary across studies but most show modest benefit for psychosis and agitation with an acceptable side effect profile. Atypical antipsychotic in general appear to have some impact in reducing psychosis as well as agitation in AD with the best evidence base for risperidone. Carefully monitored and relative brief courses of antipsychotics are recommended. Recent studies with pimavanserin addressing psychosis in the course of different types of dementia ( including Alzheimer’s disease) are showing promising results [41].
Agitation in the Course of AD
Agitation as a symptom of AD is common (prevalence ranges from 20% to 60% of) and highly disruptive. It is considered the most problematic symptoms among NPS [42]. Agitation commonly clusters with aggressive behavior and tends to co-occur with sleep disorders, delusions, hallucinations, anxiety and dysphoria [12,42]. Table 3 provides the International Psychogeriatric Association (IPA) criteria for the definition of agitation in cognitive impairment [43] (Table 3). Frontal-subcortical and corticocortical networks dysfunction is proposed as of the basis for the agitation syndrome [6].
Table 3. International Psychogeriatric Association definition of agitation in cognitive impairment.
Note: Adapted from: Cummings J, Mintzer J, Brodaty H, Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int Psychogeriatr 2015; 27:7-17.
Rationale for Interventions and Treatment
The symptom of agitation may be caused by pain or any other discomfort, medical comorbidities, environmental factors or drug effects. The optimal approach to treating agitation in AD thus requires assessing the individual patient’s medical circumstances with careful verification of medications. This step should be followed by thorough assessment of symptom severity, cognitive function, and presence of other NPS. Additionally, vulnerability to adverse effects of pharmaceuticals should be carefully assessed and included to the treatment plan. Nonpharmacological, behavioral interventions are crucial in the management of agitation in AD and are considered first-line treatments. Meta-analysis of various psychological approaches found that behavior management therapies, care by specific types of caregiver and residential care, and staff education had the most lasting benefits for the management of agitation in dementia patients. Music therapy and sensory stimulation had positive but short-lived effects [44]. In cases when psychosocial approaches are inadequate, antipsychotics, antidepressants, anticonvulsants, and other classes of drugs are being used off- label.
AChEIs studies provide only modest evidence to support benefit from their use in managing agitation [15,45]; (Howard et al.). In clinical practice AChEIs are not helpful when immediate intervention is required [6]. A study comparing galantamine, (an AChEI) with risperidone, showed that the levels of agitation decreased in both treatment groups, but the improvement was significantly greater in the risperidone group [46]. Memantine provides modest benefit in the treatment of agitation and aggression in dementia and is well tolerated. In pooled, retrospective analysis of data from three placebo-controlled trials in moderate to severe AD, memantine showed significant reduction in agitation, aggression or psychosis [35]. Substantial atypical antipsychotics are used off label despite modest clinical benefits and side-effect burden and risk of mortality. Atypical antipsychotic were more beneficial than placebo and were associated with decreases in caregiver burden, but adverse effects limit their overall effectiveness [36].
There is some evidence to support the use of typical antipsychotics to manage aggression and agitation in the acute clinical setting. Haloperidol is useful in treatment of aggression with agitation (but not general agitation behaviors, such as wandering or verbal agitation) [47]. The use of typical antipsychotics in NDD even in acute situations is considered high risk. Typical antipsychotics are not recommended in non-emergent treatment of agitation in dementia [48]. Experts recommend that risperidone, olanzapine and aripiprazole be used for severe agitation, aggression and psychosis associated with AD where there is risk of harm to the patient and/or others [13]. The potential benefit of all antipsychotics must be weighed against the significant risks, such as cerebrovascular adverse events and mortality. A metanalysis of four large placebo-controlled clinical trials proved risperidone’s efficacy in the management of agitation and aggression even in severely impaired AD patients [49]. Risperidone may be considered as an option for short term intervention in cases of acute, treatment resistant cases of agitation in AD. In AD patients with psychosis or agitation who had responded to risperidone therapy for 4 - 8 months, discontinuation of risperidone was associated with an increased risk of relapse [50].
If there is no clinically significant response after a 4-week trial of an adequate dose of an antipsychotic drug, the medication should be tapered and withdrawn. In cases of adequate response an attempt to taper and withdraw the drug should be made within 4 months of initiation, unless the patient experienced a recurrence of symptoms with prior attempts at dose reduction. There is modest evidence to support effectiveness of carbamazepine in targeting agitation and aggression in AD [16]. In practice it’s use is limited by the risk ofr common side effects such as dizziness, sedation, ataxia, confusion, headaches, nausea, vomiting, diarrhea, blurred vision; and the more rare but significant adverse effects of inappropriate antidiuretic hormone with hyponatremia, cardiac and hepatotoxicity, and increased risk of suicidal behavior and ideation [8] Patients should also be explicitly informed of warnings for aplastic anemia, agranulocytosis, and rare but sometimes fatal dermatologic adverse reactions. The evidence for valproate in management of cognition in AD is mixed, with a meta-analysis of pooled results concluding valproate is and is associated with unacceptable rates of adverse events, notably sedation and urinary tract infections [13].
Among other pharmaceuticals from this category topiramate has some efficacy; gabapentin and lamotrigine, oxycarbamazepine and levetiracetam have been the subject of observational or uncontrolled studies and are considered as low priority agents. Trazodone, a hypnotic and antidepressant (pharmacologically serotonin antagonist and reuptake inhibitor), is used for management of irritability, agitation and aggression in AD. Trazodone has sedating properties with minimal anticholinergic activity. Trazodone has a favorable safety profile if administered in small doses and appears to produce a stabilization of the circadian rhythms in individuals with AD [51]. y A few retrospective or observational studies suggest that trazodone may be effective for the treatment of aggression or agitation in AD [52]. The most promising potential pharmacological alternatives to antipsychotics and anti-epileptic agents include citalopram, dextromethorphan/quinidine, and prazosin [53]. Comparator studies indicate sertraline and citalopram are probably as effective as risperidone in treating agitation in dementia. A recent study had shown that dextromethorphan/quinidine significantly improved AD-associated agitation, reduced caregiver burden, and was generally well tolerated [42,54-60].
References
- Burns A, Jacoby R, Levy R (1990) Psychiatric phenomena in Alzheimer’s disease. Br J Psychiatry 157: 72-76.
- Cummings JL (1997) The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 48(5 Suppl 6): S10-16.
- Steinberg M, Shao H, Zandi P, Lyketsos CG, Welsh Bohmer KA, et al. (2008) Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: The cache county study. Int J Geriatr Psychiatry 23(2): 170-177.
- Steele C, Rovner B, Chase GA, Folstein M (1990) Psychiatric symptoms and nursing home placement of patients with Alzheimer's disease. Am J Psychiatry 147(8): 1049-1051.
- Lanari A, Amenta F, Silvestrelli G, Tomassoni D, Parnetti L (2006) Neurotransmitter deficits in behavioral and psychological symptoms of Alzheimer’s disease. Mech Ageing Dev 127(2): 158-165.
- Panza F, Solfrizzi V, Seripa D, Imbimbo BP, Santamato A, et al. (2015) Progresses in treating agitation: a major clinical challenge in Alzheimer's disease. Expert Opin Pharmacother 16(17): 2581-2588.
- Cummings J, Lai TJ, Hemrungrojn S, Mohandas E, Yun Kim S, Nair G, et al. (2016) Role of Donepezil in the Management of Neuropsychiatric Symptoms in Alzheimer's Disease and Dementia with Lewy Bodies. CNS Neurosci Ther 22(3): 159-166.
- Rothenberg KG, Wiechers IR (2015) Antipsychotics for Neuropsychiatric Symptoms of Dementia-Safety and Efficacy in the Context of Informed Consent. Psychiatric Annals 45(7): 348-353.
- Kales HC, Gitlin LN, Lyketsos CG (2014) Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc 62(4): 762-769.
- Cummings J, Mintzer J, Brodaty H, Sano M, Banerjee S, et al. (2015) Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int Psychogeriatr 27(1): 7-17.
- Brodaty H, Arasaratnam C (2012) Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry 169(9): 946-953.
- Cohen Mansfield J (2001) Nonpharmacologic interventions for inappropriate behaviors in dementia: a review and critique. Am J Geriatr Psychiatry 9(4): 361-381.
- Herrmann N, Lanctôt KL, Hogan DB (2013) Pharmacological recommendations for the symptomatic treatment of dementia: the Canadian Consensus Conference on the Diagnosis and Treatment of Dementia 2012. Alzheimers Res Ther 5(Suppl 1): S5.
- Olin JT, Schneider LS, Katz IR, Meyers BS, Alexopoulos GS, et al. (2002) Provisional diagnostic criteria for depression of Alzheimer disease. Am J Geriatr Psychiatry 10(2): 125-128.
- Teng E, Ringman JM, Ross LK, Mulnard RA, Dick MB, et al. (2008) Diagnosing depression in Alzheimer disease with the national institute of mental health provisional criteria. Am J Geriatr Psychiatry 16(6): 469-477.
- Ballard C, Margallo Lana M, Juszczak E, Douglas S, Swann A, et al. (2005) Quetiapine and rivastigmine and cognitive decline in Alzheimer's disease: randomised double blind placebo controlled trial. BMJ 330(7496): 874.
- Cummings JL, McRae T, Zhang R (2006) Effects of donepezil on neuropsychiatric symptoms in patients with dementia and severe behavioral disorders. Am J Geriatr Psychiatry 14(7): 605-611.
- Holmes C, Wilkinson D, Dean C, Vethanayagam S, Olivieri S, et al. (2004) The efficacy ofdonepezil in the treatment of neuropsychiatric symptoms in Alzheimer disease. Neurology 63(2): 214-219.
- Rabins PV, Blacker D, Rovner BW, Rummans T, Schneider LS, et al. (2010) Practice guideline for the treatment of patients with Alzheimer’s Disease and other dementias, (2nd).
- Lyketsos CG, Olin J (2002) Depression in Alzheimer's disease: overview and treatment. Biol Psychiatry 52(3): 243-252.
- Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, et al. (1999) Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Risperidone Study Group. J Clin Psychiatry 60(2): 107-115.
- Street JS, Clark WS, Gannon KS, Cummings JL, Bymaster FP, et al. (2000) Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. The HGEU Study Group. Arch Gen Psychiatry 57(10): 968-976.
- Tariot PN, Erb R, Podgorski CA, Cox C, Patel S, et al. (1998) Efficacy and tolerability of carbamazepine for agitation and aggression in dementia. Am J Psychiatry 155(1): 54-61.
- Hausner L, Damian M, Sartorius A, Frolich L (2011) Efficacy and cognitive side effects of ECT in depressed elderly inpatients with co-existing mild cognitive impairment or dementia. J Clin Psychiatry 72(1): 91-97.
- Miller M, Reynolds CF (2007) Expanding the usefullness of interpersonal psychotherapy (IPT) for depressed elders with co-morbid cognitive impairment. Int J Geriatr Psychiatry 22(2): 101-105.
- Teri L, Gallagher Thompson D (1991) Cognitive-behavioral interventions for treatment of depression in Alzheimer’s patients. Gerontologist 31(3): 413-416.
- Kiosses DN, Arean PA, Teri L, Alexopoulos GS (2010) Home-delivered problem adaptation therapy (PATH) for depressed, cognitively impaired, disabled elders: a preliminary study. Am J Geriatr Psychiatry 18(11): 988-998.
- Marin RS, Biedrzycki RC, Firinciogullari S (1991) Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 38(2): 143-162.
- Mulin E, Leone E, Dujardin K, Delliaux M, Leentjens A, et al. (2011) Diagnostic criteria for apathy in clinical practice. Int J Geriatr Psychiatry 26(2):158-165.
- Herrmann N, Rothenburg LS, Black SE, Ryan M, Liu BA, et al. (2008) Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J Clin Psychopharmacol 28(3): 296-301.
- Rosenberg PB, Lanctôt KL, Drye LT, Herrmann N, Scherer RW, et al. (2013) Safety and efficacy of methylphenidate for apathy in Alzheimer's disease: a randomized, placebo-controlled trial. J Clin Psychiatry 74(8): 810-816.
- Paulsen JS, Salmon DP, Thal LJ, Romero R, Weisstein Jenkins C, et al. (2000) Incidence of and risk factors for psychosis of Alzheimer’s disease. Neurology 54(10): 1965-1971.
- Jeste D, Finkel S (2000) Psychosis of Alzheimer's Disease and Related Dementias: Diagnostic Criteria for a Distinct Syndrome. American Journal of Geriatric Psychiatry 8(1): 29-34.
- D'Onofrio G, Panza F, Sancarlo D, Paris FF, Cascavilla L, et al. (2016) Delusions in Patients with Alzheimer's Disease: A Multidimensional Approach. J Alzheimers Dis 51(2): 427-437.
- Wilcock GK, Ballard CG, Cooper JA, Loft H (2008) Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer's disease: a pooled analysis of 3 studies. J Clin Psychiatry 69(3): 341-348.
- Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, et al. (2006) Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med 355(15): 1525-1538.
- Mahmoud F, Tampi RR (2011) Valproic acid-induced parkinsonism in the elderly: a comprehensive review of the literature. Am J Geriatr Pharmacother 9(6): 405-412.
- Moretti R, Torre P, Antonello RM, Cazzato G, Griggio S, et al. (2003) Olanzapine as a treatment of neuropsychiatric disorders of Alzheimer's disease and other dementias: a 24-month follow-up of 68 patients. Am J Alzheimers Dis Other Demen 18(4): 205-214.
- Mintzer JE, Tune LE, Breder CD, Swanink R, Marcus RN, et al. (2007) Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am J Geriatr Psychiatry 15(11): 918-931
- El Saifi N, Moyle W, Jones C, Tuffaha H (2016) Quetiapine safety in older adults: a systematic literature review. J Clin Pharm Ther 41(1): 7-18.
- Cummings J, Ballard C, Tariot P, Owen R, Foff E, et al. (2018)Pimavanserin: Potential Treatment For Dementia-Related Psychosis.J Prev Alzheimers Dis 5(4):253‐258.
- Soto M, Andrieu S, Nourhashemi F, Ousset PJ (2015) Medication development for agitation and aggression in Alzheimer disease: review and discussion of recent randomized clinical trial design. Int Psychogeriatr 27(2): 181-197.
- Cummings J, Lyketsos C, Tariot P (2015) Dextromethorphan/Quinidine (AVP-923) efficacy and safety for treatment of agitation in persons with Alzheimer’s Disease: Results from a Phase 2 Study (NCT01584440) (S16.007). Neurology 84(14 Suppl): 16.007.
- Livingston G, Kelly L, Lewis Holmes E, Baio G, Morris S, et al. (2014) A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess 18(39): 1-226.
- Gauthier S, Cummings J, Ballard C, Brodaty H, Grossberg G, et al. (2010) Management of behavioral problems in Alzheimer's disease. International psychogeriatrics 22(3): 346-372.
- Freund Levi Y, Jedenius E, Tysen Bäckström AC, Lärksäter M, Wahlund LO, et al. (2014) Galantamine versus Risperidone treatment of neuropsychiatric symptoms in patients with probable dementia: an open randomized trial. Am J Geriatr Psychiatry 22(4): 341-348.
- Lonergan E, Luxenberg J, Colford J (2002) Haloperidol for agitation in dementia. Cochrane Database Syst Rev (2): CD002852.
- (2016) APA Practice Guideline Writing Group’s “Practice Guideline on the Use of Antipsychotics to Treat Agitation or Psychosis in Patients With Dementia”.
- Katz I, de Deyn PP, Mintzer J, Greenspan A, Zhu Y, et al. (2007) The efficacy and safety of risperidone in the treatment of psychosis of Alzheimer's disease and mixed dementia: a meta-analysis of 4 placebo-controlled clinical trials. Int J Geriatr Psychiatry 22(5): 475-84.
- Devanand DP, Mintzer J, Schultz SK, Andrews HF, Sultzer DL, et al. (2012) Schultz SK, Relapse risk after discontinuation of risperidone in Alzheimer’s disease. N Engl J Med 367(16): 1497-1507.
- Grippe TC, Gonçalves BS, Louzada LL, Quintas JL, Naves JO, et al. (2015) Circadian rhythm in Alzheimer disease after trazodone use. Chronobiol Int 32(9): 1311-1314.
- Kitamura Y, Kudo Y, Imamura T (2006) Trazodone for the treatment of behavioral and psychological symptoms of dementia (BPSD) in Alzheimer's disease: a retrospectivestudy focused on the aggression and negativism in caregiving situations. No To Shinkei 58(6): 483-488.
- Antonsdottir IM, Smith J, Keltz M, Porsteinsson AP (2015) Advancements in the treatment of agitation in Alzheimer's disease. Expert Opin Pharmacother 16 (11): 1649-1656.
- Marsh L, Williams JR, Rocco M, Grill S, Munro C, et al. (2004) Psychiatric comorbidities in patients with Parkinson disease and psychosis. Neurology 63(2): 293-300.
- Marsh L, McDonald WM, Cummings J, Ravina B, NINDS/NIMH Work Group on Depression, et al. (2006) Provisional diagnostic criteria for depression in Parkinson's disease: report of an NINDS/NIMH Work Group. MovDisord 21(2): 148-158.
- Menza MA, Robertson-Hoffman DE, Bonapace AS (1993) Parkinson’s disease and anxiety: comorbidity with depression. Biol Psychiatry 34(7): 465-470.
- Pact V, Giduz T (1999) Mirtazapine treats resting tremor, essential tremor, and levodopa-induced dyskinesias. Neurology 53(5): 1154.
- Reisberg B, Borenstein J, Salob SP, Ferris SH, Franssen E, et al. (1987) Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment. J Clin Psychiatry 48 Suppl: 9-15.
- Rektorova I, Balaz M, Svatova J, Zarubova K, Honig I, et al. (2008) Effects of ropinirole on nonmotor symptoms of Parkinson’s disease: a prospective multicenter study. Clin Neuropharmacol 31(5): 261-266.
- Workman RH Jr, Orengo CA, Bakey AA, Molinari VA, Kunik ME (1997) The use of risperidonefor psychosis and agitation in demented patients with Parkinson sisease. J Neuropsychiatry Clin Neurosci 9(4): 594-597.

 Review Article
Review Article