Abstract
Background: Neuroblastoma is the highest mortality rate extracranial soild tumor in childhood. Accumulating evidence indicated that long noncoding RNAs (lncRNAs) are widely expressed in neuroblastoma, and playing an important role in the development and progression.
Methods: RNA sequencing was conducted to identify differentially expressed lncRNAs in four Ⅲ phase and four Ⅳ phase tumor tissues of neuroblastoma. RT-qPCR was carried out to validate the result of sequencing. Clinical information was reviewed to analyze the relationship between lncRNA and clinical characteristics. The public database R2 was used to analyze prognosis.
Result: Differentially expressed lncRNAs were identified. LRRC75A-AS1 was the overexpressed lncRNA in Ⅳ phase patients. RT-qPCR was conducted in tumor tissues, confirming the tendency with sequencing. And higher expression of LRRC75A-AS1 was associated with N-MYC (p < 0.001), advanced stage (p = 0.029), Risk group (p = 0.027). Furthermore, LRRC75A-AS1 was correlated with Shimada classification(p = 0.046), LDH level (r = 0.390, p = 0.003), D-Dimer level (r = 0.338, p = 0.012) , and NSE level (r = 0.284, p = 0.05). The neuroblastoma dataset shows that patients with overexpressed LRRC75A-AS1 have a worse prognosis than down-expressed.
Conclusion: LRRC75A-AS1 is associated with clinical characteristics of neuroblastoma and may function as a prognostic predictor or a therapeutic target.
Keywords: Biomarker; lncRNA LRRC75A- AS1; Neuroblastoma
Abbreviations: NB: Neuroblastoma; lncRNAs: Long Noncoding RNAs; LDH: Lactate Dehydrogenase; NSE: Neuron-Specific Enolase; ORFs: Open Reading Frames; uFH: Unfavorable Histology; FH: Favorable Histology; NA: Not Amplified; NM: Not Metastasis; PD: Progressive Disease; VMA: Vanilla Mandelic; SNHG29: Small Nucleolar RNA Host Gene 29; TJ: Tight Junction; CRC: Colorectal Carcinoma
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common issue
of Idiopathic Interstitial Pneumonia (IIP) and causes progressive
pulmonary fibrosis. Symptoms (exertional dyspnea, non-productive
cough, and ‘Velcro’ lung crackles) tend to increase progressively.
The diagnosis of IPF is based on the clinical picture and High-
Resolution CT Scans (HRCT) or lung biopsy, when needed. Currently,
treatment with antifibrotic drugs and oxygen therapy may help
relieving symptoms but it is not clear if the progression can be
changed. Most patients show a progressive degeneration of the
lungs with a median survival (possibly, based on initial conditions,
management, and age) from 3 to 5 years [1-5]. Histology shows
interstitial pneumonia in most cases. Environmental, genetic, and
unknown factors contribute to alveolar epithelial cells dysfunction
or reprogramming leading to the abnormal fibroproliferation in
the lungs. The key histological findings (subpleural fibrosis with
fibroblast foci, sites of dense proliferation and intense scarring),
alternating with areas of apparently normal tissue are visible with
a diffuse heterogeneity of the tissues. Scattered areas of interstitial
inflammation (with lymphocytes and plasma cells) tend to increase
in time. Accessory symptoms (low-grade fever or myalgias) are
uncommon. Clubbing can be seen in some 50% of the cases.
A classic sign is the presence of fine dry inspiratory crackles
(Velcro) at both bases of the lungs. Most blood tests tend to be
normal. More severe signs (pulmonary hypertension and right
ventricular dysfunction) may develop [1-7]. IPF is often overlooked
or confused with bronchitis, asthma, or heart failure. Diagnosis is
made with High-Resolution Computer Tomography (HRCT). Chest
x-ray shows diffuse reticular opacities in the lower-peripheral
zones, small cystic lesions (caused by honeycombing), and some
dilated airway (traction bronchiectasis). HRCT shows diffuse,
patchy, subpleural, reticular opacities in the lower and peripheral
lung zones. Ground-glass opacities (in more than 30% of the
lungs) tend to suggest a different diagnosis. Lab tests are basically
within normal values in most patients. Lung’s health of most
subjects tends to deteriorate in a relatively short period of time.
The worst prognosis is related to older age, male sex, lower forced
vital capacity and lower diffusing capacity of the lung for carbon
monoxide or CO (DLCO also known as TLCO). DLCO is the extent to which
oxygen passes from the air sacs of the lungs into the bloodstream.
This refers to the test commonly used to evaluate this parameter.
Acute problems (infections, embolism, pneumothorax, and
heart failure) may cause acute lung deterioration. This condition
has a high mortality and morbidity: lung cancer is more frequent
in these patients for concomitant risks, but the causes of death are
associated with respiratory failure [6,7]. The aim of this study was
the evaluation of the supplementary activity of the combination
of Pycnogenol® and Centella asiatica (as Centellicum®) (PY-CE) in
subjects with IIP progression. During the recent period, a significant
problem (the post-COVID-19 lung disease) has emerged with very
large numbers. Considering the antifibrotic characteristics of the
combination PY-CE (effective in blocking the passage from edema
to fibrosis, in different tissues), we also tested this supplementary
management in post-COVID-19 patients [8].
Methods
Part I Idiopathic Interstitial Pneumonia
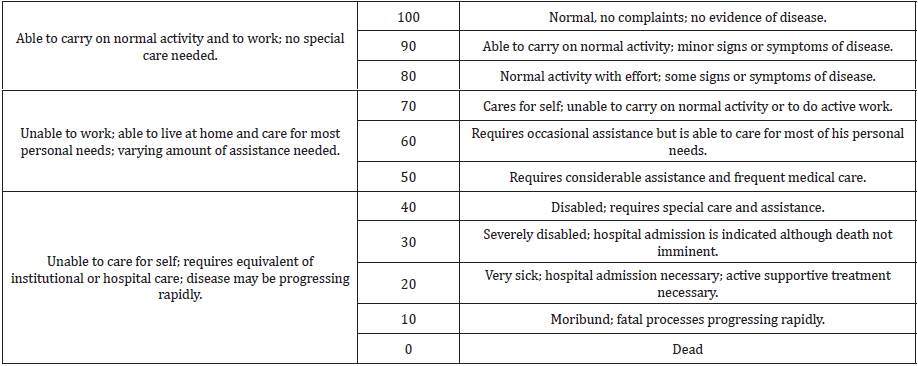
Patients included: subjects with Idiopathic Interstitial Pneumonia (IIP) were included. The subjects had a Karnofsky performance scale index from 80 to 60 (Table 1). Only males aged <65 were included. No other disease was present. No drugs were used excluding specific management for IFP, i.e. pirfenidone. No metabolic conditions were present, and the participants’ BMI was under 26. The subjects with IIP were in management for fast growing atherosclerotic carotid plaques with no surgical indications, using Pycnogenol® and Centellicum® according to our previous studies. In these subjects, fibrosis of the lung was a ‘secondary’ problem and the effects on the lungs were studied as a corollary evaluation to the initial management of atherosclerosis. The main target was the evaluation of changes in the Karnofsky performance scale. Cardiac Ultrasound showed no signs of significant pulmonary hypertension. There was a good left ventricular function. There were no valvular abnormalities.
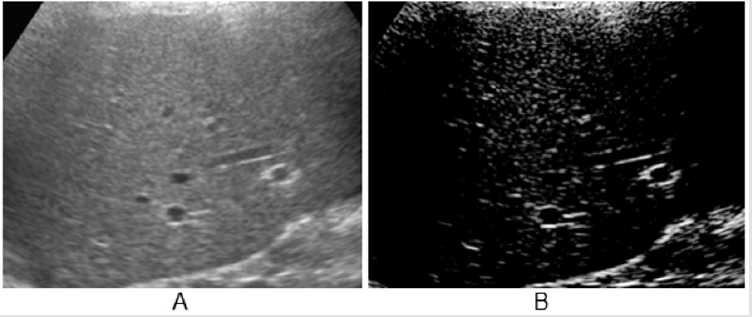
Figure 2: A: Aspect of HR scan, lung ‘slice’ (Preirus, Hitachi).
B: High-density white components in normal lung (<8% of the total image).
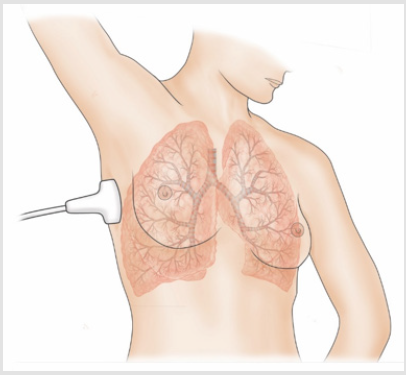
Lung Ultrasound: a section of lung is imaged (Figure 1) in a vertical scan. The image is elaborated (Adobe Premiere) for white components (speckles), corresponding to the most fibrotic components and areas of fibrosis in a normalized grey scale. The grey scale median is an expression of the white components (fibrotic, associated with collagen) in the ultrasound section. The section is obtained with a vertical, trans axillary scan (arm elevated). The ‘slices’ [4] are scanned for fibrotic components and for vascularization. The content of white ‘speckles’ corresponds to the presence of fibrotic elements. In a normal lung section of this type, the white components are about 8% of the section or less. Figure 2 shows an elaboration of a ‘slice’ of lung tissue with its ‘white’ components corresponding to high collagen level (full black is the density of blood). The same software is used to image the arterial plaques to define their content in fibrotic (white) components or i.e., thrombotic (black on ultrasound scans, same density of blood). Supplement study: This study (and the subsequent post-COVID-19 lung study) were conducted as supplement registry studies [8,9]. The role of the combination Pycnogenol®-Centellicum® to control fibrosis has been previously defined in several clinical models [10]. 150 mg/day of Pycnogenol® was administered in 3 doses and Centellicum® (centella asiatica) was given at the doses of 225 mg 3 times per day. Both supplements are produced by Horphag Research.
Statistics [11-13]. A number of at least 15 subjects for each group (SM and SM + supplementation) was considered necessary to evaluate differences in target parameters over 4 weeks. All results and data were considered as non-parametric; the Mann-Whitney U-test and the ANOVA were used for symptoms/complaints. A predictive analysis [14] was performed at the end of the study based on the observed data and results.
Part II: Post-COVID-19 Lung Disease
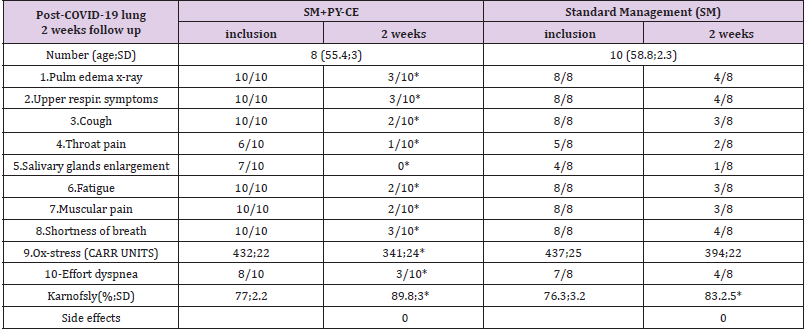
In an additional evaluation, subjects with Post-COVID-19 Lungs Disease (PCL), a second group of subjects (the study is in progress) were included in a registry; these were previously symptomatic patients, briefly admitted into hospitals (no ICU) and then sent home with some signs/symptoms (under control) with a standard management, based on the controls of the most common symptoms. A number of these subjects (all males) had also used as a supplementary management the combination of Pycnogenol® (150 mg/day in 3 doses) and Centellicum® (Centella asiatica, at the doses of 225 mg 3 times/daily). They were all using the supplementation before being diagnosed with COVID-19 and hospital admission, to slow down the progression of atherosclerotic, carotid plaques. Standard management was based on anti-inflammatory drugs; aerosols (no routine corticosteroids); antibiotics if indicated; vitamins, electrolytes, mild respiratory exercise (Triflo); appropriate diet (including proteins). Other drugs, like corticosteroids or cough meds, were used as needed. Table 3 shows the most common symptoms and the Karnofksy performance scale index in 2 weeks of observation or supplementation.
Results
Part I. Idiopathic Interstitial Pneumonia
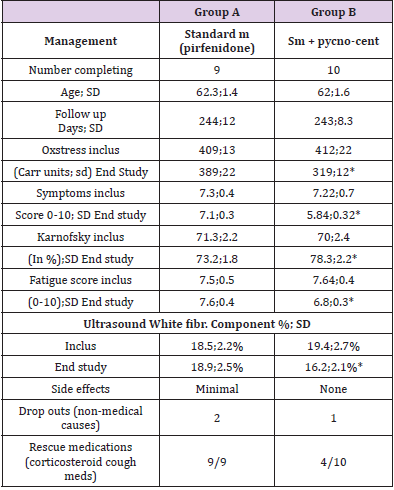
19 subjects with Idiopathic Interstitial Pneumonia were included in the study. 10 were treated with the Pycnogenol® Centellicum® combination and 9 served as controls. No side effects were seen for the supplementation. A good tolerability was observed. Table 2 shows the two main groups of subjects. They were comparable at inclusion. Oxidative stress was very high in all subjects at inclusion and decreased significantly after 8-month treatment in the supplement group (p<0.05). The Karnofsky scale significantly improved in the supplement group in comparison with controls (p<0.05). The symptoms, fatigue and muscular pain were significantly lower at the end of the study in all supplemented patients (p<0.05). As inclusion criteria, HRCT had been indicative of fibrosis. Chest x-ray had shown diffuse reticular opacities at inclusion (mainly at the lower-peripheral zones). Diffuse, small cystic lesions (defined as honeycombing) had been observed. Some dilated airways (traction bronchiectasis) had been detected. CT had also shown diffuse, patchy, subpleural, reticular opacities (at the lower and peripheral lung zones). At the end of the study, the small cystic lesions (characteristic of honeycombing) and the traction bronchiectasis were stable or in partial regression in 4 subjects in the supplemented group (vs none in the control group) with a significant improvement in tissue edema in supplemented subjects (in 8 out of 10 patients) compared to the control group (1 out of 9 patients). Ultrasound Lung Sampling: The white fibrotic component at inclusion was 18.5±2.2% of the image in controls vs 19.4±2.7% in the supplement group (ns). At the end of the study, there was no improvement in controls (18.9±2.5%) vs a significant improvement (decrease) in the supplemented subjects (16.2±2.1%; p<0.05). Rescue medication (including corticosteroids) were used significantly less (p<0.05) in the supplement group.
Table 2: Summary Table of the idiopathic interstitial pneumonia (IIP) subjects.
VARIATION OF THE MOST IMPORTANT ITEMS. *=p<0.05 vs controls
Comments
IPF is the most common of IIPs. Symptoms are often misleading
and may suggest other diseases [15].Therapy is complex and
difficult: Pirfenidone and nintedanib are available for treatment
in selected patients. These drugs tend to slow the progression of
fibrosis and control symptoms down. However, they often cause
side effects and costs are still high. Pirfenidone reduces lung fibrosis
through downregulation of the production of growth factors and
procollagens I and II. The recommended dose of pirfenidone in
patients with idiopathic pulmonary fibrosis (as in the subjects in
this registry) is 801 mg, 3 times a day (total daily dose of 2,403
mg). Supportive measures (oxygen and physical therapy) also help
to control the symptoms as well as nutrition and weight and stress
control. The use of prostaglandin E1 is supportive as it improves
the pulmonary microcirculation, relieving vasospasm and increases
the exchange rate of O2 and CO2 at bronchiolar levels with a more
dynamic perfusional circulation, within the same limited, reduced
exchange surface.
The evaluation of the pulmonary circulation is an important
issue as a better gas exchange can be obtained with a better
perfusion even in subjects suffering from severe fibrosis. The role of
oxygen in promoting fibrosis must be evaluated. Cardiac ultrasound
is especially important. Alterations (including dilatation) in the
right ventricle volume indicate severe pulmonary conditions and a
possible fast deterioration. When the right ventricle becomes larger,
pulmonary hypertension is involved and a negative, fast evolution is
possible. Most of these patients benefit from PGE1 infusions as they
are also vascular subjects. The use of prostaglandin – off label – in
these patients, to increase pulmonary circulation, does not produce
side effects and it is well tolerated.
Part II. The Post-Covid-19 Lung (PCL) Experience
Preliminary results (Table 3) 18 subjects with post-COVID-19 lung disease were included in the study. 10 were treated with the Pycnogenol® Centellicum® combination and 8 served as controls. Supplementation did not cause side effects and was well tolerated. The preliminary results on symptoms associated with post- COVID-19 Lung disease after 2 weeks were significantly improved with the supplement combination as compared with baseline. Oxidative stress and the Karnofsky performance scale were significantly improved in the supplements group as compared with controls (p<0.05). Morphological CT or ultrasound scans will show (the study is in progress) the evolution of the tissue scarring, but over a longer period of time.
Table 3: Subjects evaluated 2 weeks after hospital exit (with manageable, not life-threatening symptoms).
*=p<0.05
Conclusion
According to these observations, Pycnogenol® helps to control and decrease edema in several conditions and Centellicum® (Centella asiatica)- modulating the apposition of collagen in the affected tissues–possibly, slows down or modulates the development of irregular cicatrization, keloidal scarring and fibrosis [9-10,15]. More time and specific evaluation methods [16] are needed to evaluate this effect and a larger number of PCL patients may be considered for a disease that has affected almost 5 million subjects worldwide so far, leaving severe consequences. Pycnogenol® and Centellicum® may improve the residual clinical picture in PCL and, in the long-term reduce the number of subjects progressing to lung fibrosis [8,16]. The key passage and evolution from edema to fibrosis seems to be slower or attenuated with this safe supplement combination both in IPF and in PCL.
References
- CrooksV, Waller S, T Smith, T J Hahn (1991) The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol46(4): M139-M144.
- De Haan R, Aaronson A, M Limburg, R L Hewer, H van Crevel (1993) Measuring quality of life in stroke. Stroke24(2):320-327.
- Hollen PJ, Gralla RJ, M G Kris, C Cox, C P Belani, et al. (1994) Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies Psychometric Assessment of the Lung Cancer Symptom Scale. Cancer73(8): 2087-2098.
- OToole DM, Golden AM (1991) Evaluating cancer patients for rehabilitation potential. West J Med155(4):384-387.
- (1993) Oxford Textbook of Palliative Medicine. Oxford University Press.
- Schag CC, Heinrich RL, Ganz PA (1984) Karnofsky performance status revisited: Reliability, validity, and guidelines. J Clin Oncology2(3):187-193.
- King TE, Bradford WZ, CastroBernardini S, Fagan EA, Glaspole I,et al. (2014) A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med370(22):2083-2092.
- Rochwerg B, Neupane B, Zhang Y, Garcia CC, Raghu G, et al. (2016) Treatment of idiopathic pulmonary fibrosis: a network meta-Analysis.BMC Med14:18.
- Belcaro G, Cornelli U, Cesarone MR, Feragalli B, Bombardelli E, et al. (2020) Seven immediate, low-cost management strategies for Covid. Exploiting viral Thermolabity: Possible, immediate solutions. Biomed Journal of Science Technical Res27(3): 20801-20808.
- Belcaro G (2017) PS SupplementsClinical applications. Imperial College Press-World Scientific Publications.
- Belcaro G, Ippolito E, Dugall M, Hosoi M, Cornelli U, et al. (2015) Pycnogenol® and Centella asiatica in the management of asymptomatic atherosclerosis progression.Int Angiol34(2):150-157.
- Maxwell C (1987) Cambridge Med Publications Cambridge.
- Cox (1992) Planning of experiments. J Wiley & Sons 320.
- Sielel (2013) Predictive Analytics.J Wiley&Sons.
- Porter R, Kaplan J, Lynn R, Reddy M (2019) The Merck Manual of diagnosis and Therapy.
- Klok FA, Boon GJAM, Barco S, Endres M, Geelhoed JM,et al. (2020) The Post-COVID-19 Functional Status (PCFS) Scale: a tool to measure functional status over time after COVID-19. Eur Respir J.

 Review Article
Review Article