Abstract
Thermal transport properties of substances are fundamental for science and engineering. Among these properties, thermal diffusivity characterizes the way materials transport heat under non-stationary conditions. The manner in which this thermal property behaves with the size or molecular configuration is very important to understand the mechanisms involved in heat transport. It is a complicated work to study variations on this thermal property taking these two variables (size and configuration) together. Linear alkanes have essentially the same spatial configuration, varying only the molecular size (the number of involved carbon atoms in the molecule), so they are ideal substances to study the behavior of thermal properties with the molecular size alone. In this work photopyroelectric technique, taking the sample´s thickness as variable (the so-called TWRC method), is used for thermal diffusivity measurements of linear alkanes, from 1-heptane to 1-heptadecane. It is shown that this thermal property increases with the molecular size. This behavior can be explained in a very simple way if it is considered that the increase in molecular size increases “routes” of heat transport.
Keywords: Linear Alkanes; Photothermal Techniques; Thermal Characterization
Introduction
The knowledge of thermal properties, especially for substances in liquid phase, is very important since this state of matter is widely used in industry, particularly as heat exchangers. The knowledge of these properties is also important in basic science since this information can be used to support theoretical schemes concerning molecular configurations, for instance. Under this perspective is interesting to figure out how the size or molecular configuration influences thermal properties. Photopyroelectric (PPE) techniques are now widely used for thermal characterization of liquids; this can be done by measuring thermal diffusivity, effusivity and specific heat [1-5]. PPE techniques, taking the sample´s thickness as variable (the so-called Thermal Wave Resonator Cavity (TWRC)) [4,5], provides high-precision measurements of thermal diffusivity, for which is ideal to carry out empirical studies about the influence of size or molecular configuration on this thermal property. However, the ideal situation is when this study is carry out taking only one of these two possible variables andlinearalkanes result in ideal substances since these organic molecules have a tree configuration with chemical formula CnH2n+2, where C atoms are the vertebral column of the structure. With this idea in mind, the TWRC method is used for measuring the thermal diffusivity for a series of linear alkanes, from 1-heptane to 1-heptadecane, to figure out the influence of the molecular size on this thermal property.
Theoretical Considerations
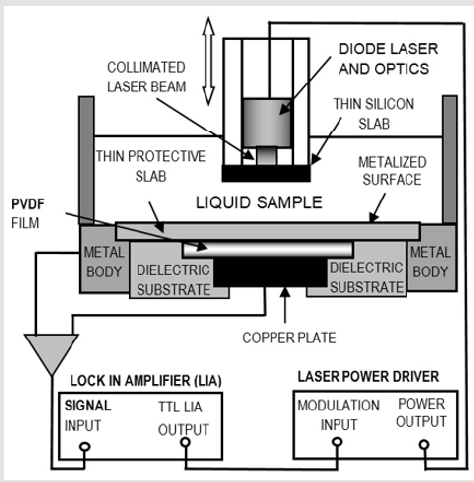
Figure 1 shows a cross section of the PPE experimental setup for thermal diffusivity measurements. Monochromatic radiation, at fix modulation frequency f, strikes a thin silicon slab; thermal waves (temperature fluctuations) generated inside this material travel to the liquid sample and reach the pyroelectric material (a thin PVDF foil). The signal is proportional to the average temperature inside the pyroelectric material. At this way solving the corresponding set of differential equations to find the temperature profile inside the pyroelectric sensor and under the appropriate experimental conditions. It can be shown that, if the liquid sample´s thickness, l, is took as variable this photopyroelectric signal takes the very simple form: V (l) = He−os , where σ = (1+ i)(π f /α )1/2 is a complex parameter involving sample´s thermal diffusivity and H is a complex expression which is independent of the sample’s thickness [4,5]. Linear fits to the amplitude and phase of this equation renders parameter M, from which sample´s thermal diffusivity can be obtained as α =π f / M2 .
Experimental Procedure
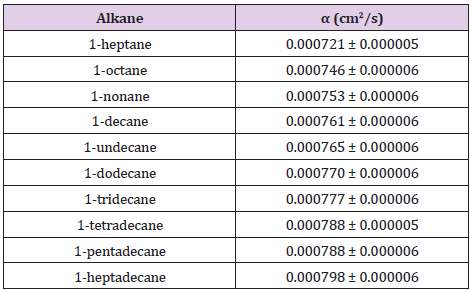
Ten linear alkanes, from 1-heptane to 1-heptadecane (Table 1), (column 1) were taken for the study. Thermal diffusivity measurements were carried out making the analysis on a set of 10 experimental data. These data consisted of PPE voltage amplitude for ten liquid sample´sthicknesses, taken at increments of 0.0050 cm. Approximately 2 ml of liquid sample was required for each thermal diffusivity measurement. Linear fits were done to obtain slopes M for each sample from which the corresponding thermal diffusivity was calculated. All measurements were made at a fix modulation frequency of 1 Hz, setting the lock-in´s time constant at 3 s and at room temperature of 23 °C.
Experimental Results
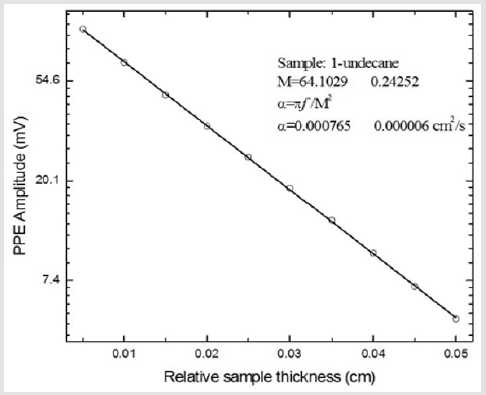
(Figure 2) shows the linear behaviour of the photopyroelectric amplitude, as a function of the sample´s thickness, for 1-undecane. The continuous line in this figure is the best linear fit as to obtain the parameter M, from which sample´s thermal diffusivity can be obtained, as it was described above. All analytical procedures were carried out using commercial analytical software (Origin 6.1TM) and the corresponding uncertainties were estimated by means of the usual formulas for error propagation. Thermal diffusivity values for all samples are summarized in (Table 1), column 2. (Figure 3) shows samples´ thermal diffusivities as a function of the molecular size. It is evident that this thermal property increases with molecular size.
Figure 2: Photopyroelectric amplitude, as a function of the sample´s thickness, for 1- undecane. The continuous line represents the best linear fit as to obtain parameter M, from which sample´s thermal diffusivity can be obtained.
Figure 3: Thermal diffusivities, as a function of the sample´s molecular weight, for the 1- alkanes studied in this work.
Conclusion
Photopyroelectric techniques (TWRC) were shown adequate
for measuring thermal properties of 1-alkanes. It was empirically
demonstrated that this thermal property increases with the
molecular size, if molecular configuration is unchanged. This
behaviour could be explained in a very simple form if it is considered
that for this kind of molecules heat transport is due to vibrations or
rotations of the C-H bonds. Increment of the molecular size increases
the number of these bonds, providing at this way more “routes” of
heat transport to the molecule and the final effect is an increment
in thermal diffusivity. Similar behaviour forthermal conductivity
could be expected, as suggested from thermal conductivities
reported for 1-butane, 1-heptane and 1-decane [6]. The monotonic
behaviour of this thermal property for 1-alkanes contrast with the
one reported for 1-alcohols [7], which are linear molecules that
differs from 1-alkanes only in an additional OH radical at one end.
Thermal diffusivity decreases in this case with the molecular
size, but this happens only for the first 5 alcohols (from methanol
to 1-pentanol) then, starting at 1-hexanol, this thermal property
increases with the increase of the molecular size. This peculiar
behaviour of thermal diffusivity for linear alcohols can be explained
as a “competition” of thermal properties between the OH- radical and the CH chain. For lineal alcohols of small size thermal diffusivity
is driven for the OH radical meanwhile that for those ones of high
molecular weight this thermal property is driven for CH bonds. In
linear alkanes, the monotonic behaviour in thermal diffusivity can
be then attributed to the thermal properties of the CH bonds and the
chain´s size. These kind of molecules (alkanes and alcohols could be
also used to figure out in what manner the molecular configuration
influences the thermal properties) and the corresponding results
could be complemented with computational simulations.
References
- Fuente R, Mendioroz A, Apiñaniz E, Salazar A (2012) Simultaneous Measurement of Thermal Diffusivity and Optical Absorption Coefficient of Solids Using PTR and PPE: A Comparison. Int J Thermophys33: 1876-1886.
- BalderasLópez JA (2007) Thermal effusivity measurements for liquids: A self-consistent photoacoustic methodology. Rev Sci Instrum 78: 064901.
- DelgadoVasallo O, Marín E (1999) The application of the photoacoustic technique to the measurement of the thermal effusivity of liquids. J Phys D: Appl Phys 32(5): 593.
- BalderasLópez JA, Mandelis A, García JA(2000) Thermal-wave resonator cavity design and measurements of the thermal diffusivity of liquids. Rev Sci Instrum 71: 2933.
- BalderasLópez JA, JaimeFonseca MR, GálvezCoyt G, MuñozDiosdado A, DíazReyes J (2015) Generalized Photopyroelectric Setup for Thermal-Diffusivity Measurements of Liquids. Int J Thermophys 36(5-6): 857-861.
- Poling Bruce E, Prausnitz John M, O’Connel John P (2001) “The properties of gases and liquids”, Fifth edition. McGraw-Hill: New York. pp. 768.
- BalderasLópez JA, JaimeFonseca MR, G GálvezCoyt, MuñozDiosdado A, Pescador JA, et al. (2018) Photothermal Techniques for Thermal Characterization of Linear Alcohols. Int J Thermophys 39: 111.

 Short Communication
Short Communication