Abstract
Introduction: Microvascular Invasion (MVI) was one of the most important factor related to postoperative tumor recurrence. Early identification of MVI before the surgery is important to make a good planning treatment for Hepatocellular Carcinoma (HCC) patients.
Materials and Methods: Retrospective clinical data from 210 patients of non-metastasis single lesion HCC patients who underwent hepatic resection surgery as the only curative treatment are collected, including general clinical data, preoperative tumor marker, inflammation response factor, other biomarker correlate with HCC and liver function, characteristic of tumor in imaging modality, and postoperative histological data. All data divided in two groups, MVI (+) and MVI (-) groups. Multivariate analysis with logistic regression are performed on statistically significant factor.
Results: The positive rate of MVI reached 29% (61/210 patients). Univariate analysis found significant difference result in tumor differentiation (P= .001), tumor size ≥3.5 cm (P= .001), tumor margin (P< .001) and alfa-fetoprotein (AFP) level ≥100 μg/L (P= .007). Multivariate logistic regression analysis shows that poorly differentiated tumor cell (OR=1.470, 95%CI: 1.040-2.077, P= .03) and tumor size ≥3.5 cm (OR=2.205, 95%CI: 1.123-4.333, P= .02) were independent risk factor of MVI.
Conclusion: Poorly differentiated tumor and tumor size ≥3.5 cm are independent risk factor of MVI and could help us to predict the possibility of MVI in non-metastasis single lesion HCC patients.
Keywords: Hepatocellular Carcinoma; Liver Cancer; Vascular Invasion; Risk Factor
Abbreviations: 18F-FDG PET/CT = Flourine- 18 Fluorodeoxyglucose Positron Emission Tomography; AFP: Alfa-Fetoprotein ALT: Alanine Transaminase; CEA: Carcinoembryonic Antigen; CECT: Contrast Enhanced Computed Tomography; CEUS: Contrast Enhanced Ultrasonography; CT: Computerized Tomography; Gd-EOB-DTPA- MRI: Gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid enhanced Magnetic Resonance Imaging HBV: Hepatitis B Virus; HCC: Hepatocellular Carcinoma; HCV: Hepatitis C Virus; INR: International Normal Ratio; MRI: Magnetic Resonance Imaging; MVI: Microvascular Invasion; PT: Prothrombin Time; ROC: Receiver Operating Characteristic; SD: Standard Deviation; SHCC: Small Hepatocellular Carcinoma
Introduction
Hepatocellular Carcinoma (HCC) is world fifth most common malignancy and third most common cause of cancer-associated death worldwide. In recent year, annual death with 700.000 was recorded around the globe [1]. Liver cirrhosis is the cornerstone of HCC in around 80% of cases [2]. Some other factors have been proved to be associated with HCC, such as Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) infection and nonalcoholic fatty liver disease [3,4]. In past several decade, clinical diagnosis and treatment of HCC has been improve, but the long-term survival rates remain unsatisfactory [5]. Previous study found that the prognosis of HCC is depend on multiple factor such as the age of the patient [6], tumor size, number of the tumor, presence of tumor vascular invasion [7,8], serum Alfa-Fetoprotein (AFP) level [9], and presence extrahepatic metastasis [10]. Tumor vascular invasion was classified as macrovascular invasion, which grossly recognizable (mostly large and medium vessels) and Microvascular Invasion (MVI), which could only found in microscopic examination (mainly in small vessels such as portal vein branches, central vein in noncancerous liver tissue, and venous vessels in tumor capsule) [11,12]. Microvascular invasion also known for negative influence outcome following surgical treatment HCC, it has been reported to be one of the most important risk factor related to postoperative early tumor recurrence [13-16]. Lim et al. [17] reported that MVI is more prominent tumor recurrence and overall survival predictor than Milan criteria for HCC after surgical resection. Patients who fulfill Milan criteria should have single tumor not more than 5 cm or three or fewer tumors with the largest tumor not exceeding 3 cm, and no evidence of macrovascular invasion or extrahepatic metastasis [7]. Lim et al. [17] also concluded that there was no significant difference in overall survival rates of patients without MVI and not exceeding the Milan criteria relative to patients within Milan criteria. However, the overall survival rate decrease significantly with MVI. Therefore, an accurate preoperative prediction of MVI is important to make a treatment planning for impeding recurrence and improving outcome of the patients. The aim of this study was to analyze the correlation between preoperative clinicopathological data of the patients with histological result in hepatic resection single lesion non-metastasis HCC patients and try to found the risk factor that could help to predict MVI.
Materials and Methods
Patient Selection
Two hundred and ten patients with non-metastatic single lesion HCC underwent hepatic resection surgery at Department of Hepatobiliary Pancreatic Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, between 2017 and 2018, based on the following criteria: (1) hepatectomy as a treatment of HCC; (2) no adjuvant treatment before hepatectomy; (3) no invasive diagnostic procedure before hepatectomy; (4) no macrovascular invasion found; (5) complete resection with incised margin was negative; (6) without lymph node and distant metastasis; (7) complete preoperative data. All the data before surgery and histological data after surgery of every patients were collected for analysis of MVI.
Data Collection
Data of the patient before and after surgery were retrospectively collected. Some general clinical data like gender, age, history of hepatitis B, history of cirrhosis and child pugh status were collected. Serum index like total bilirubin, albumin, Alanine Transaminase (ALT), Prothrombin Time (PT) and International Normal Ratio (INR) were collected. We also collect patient’s preoperative inflammation response factors like lymphocyte count, neutrophil count, platelet count, and also calculate the neutrophil lymphocyte ratio by dividing neutrophil count by lymphocyte count, and platelet lymphocyte ratio by diving platelet count by lymphocyte count, all the inflammation response factors were obtain from the same blood sample. Some tumor marker like AFP, Ca19-9, Carcinoembryonic Antigen (CEA) were collected. Preoperative conventional enhance CT (Computerized Tomography) and MRI (Magnetic Resonance Imaging) examination of every patients were evaluated, data of tumor size and tumor margin were collected. Postoperative histological data for tumor differentiation and tumor capsule were collected.
Statistics
The statistical significance of differences of selected clinicopathology features between those with MVI and without MVI, for those continues variable were expressed as means ± standard deviation (SD) and compared using Student’s t-test or Mann–Whitney test for variables with an abnormal distribution. All data will be dichotomized with a reliable cut-off value and will be presented in percentage and assessed by chi- square test. Variable at P-value < .05 on univariate analysis were subjected to multivariate analysis by fitting logistic regression model to identify independent predictors for MVI in non-metastatic single lesion HCC. The SPSS 23 statistical software was used to perform the statistical analyses. Variable with P-value < .05 was considered statistically significant.
Results
Patient Characteristics
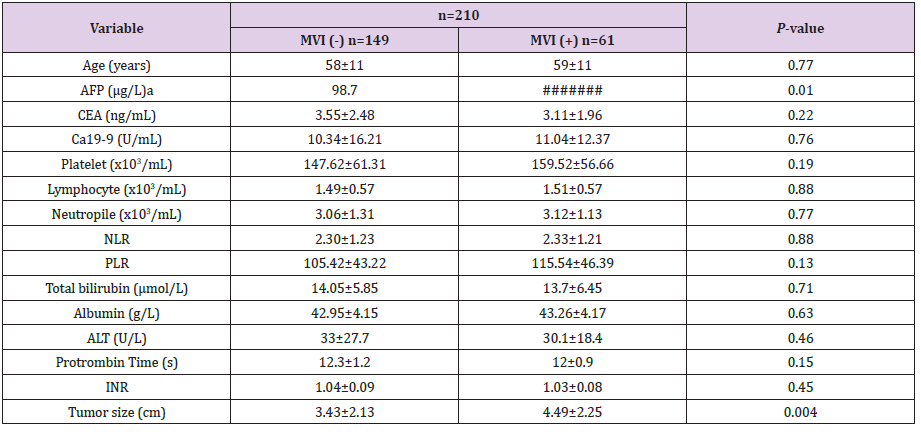
Table 1: Comparison of quantitative clinical factors between MVI (-) group and MVI (+) group.
Note: MVI: Microvascular Invasion; AFP: Alfa-Fetoprotein; CEA:
Carcinoembryonic Antigen; NLR: Neutrophil Lymphocyte Ratio;
PLR: Platelet Lymphocyte Ratio; ALT: Alanine Aminotransferase;
INR: International Normal Ratio.
aMann-Whitney test was used.
This study included 210 patients with non-metastasis single tumor HCC, including male (n=184) and female (n=26) who had undergone a curative resection for HCC. The average age of the patients was 58.53 ± 10.64 years. Microvascular invasion was found in 61 patients (29.05%) among 210 of our study patients. Most of the patient have a history of hepatitis B infection with Hepatitis B surface antigen positivity accounted for 87.1% (183/210). All diagnoses were confirmed both clinically and pathologically, and MVI was confirmed pathologically. The comparison of quantitative clinical factors between this two group summarized in Table 1. There are significant difference in AFP and tumor size variable with P-value .01 and .004, respectively. There are no significant difference between two groups in other quantitative clinical factor.
Determination of the Cut-Off Value
According to the Receiver Operating Characteristic (ROC) curve (Figure 1), the cut-off value of tumor size for microvascular invasion was set to 3.5 cm (AUC=0.626, sensitivity=0.623 and 1-specificity=0.396). Thus, the patients were dichotomized into group of tumor size larger than 3.5 cm and smaller than 3.5 cm.
Figure 1: Receiver operating characteristic (ROC) curves for HCC tumor size according to MVI-positive.
Univariate and Multivariate Analyses of MVI-Related Factors
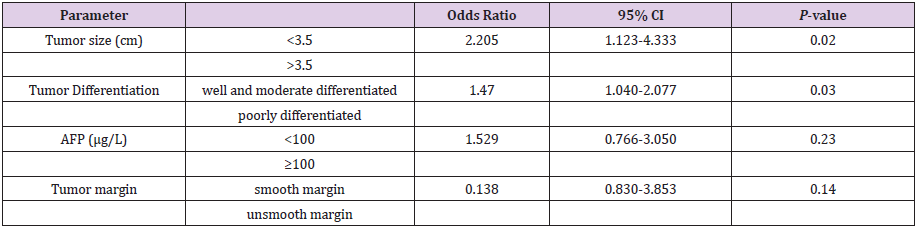
Comparisons of clinical characteristic in univariate analysis between MVI (-) and MVI (+) group are summarized in Table 2. There were no significant difference between patient with MVI and without MVI in gender, history of hepatitis B infection, and history of cirrhosis. Analysis in AFP with 100 μg/L as a cut-off found that there was significant difference between the two group (P= .007), MVI (+) group had more patient with AFP higher than 100 μg/L compare to MVI (-) group (49.2% vs 29.5%), but other tumor markers like Ca19-9 and CEA has no significant difference value. Tumor characteristic from imaging examination and histopathology found that microvascular invasion was more often found in HCC larger than 3.5 cm (P= .001), unsmooth tumor margin (P< .001) and poorly differentiated tumor cell (P= .001). However, in the evaluation of the tumor capsule there are no significant difference between the two groups (P=0.11). For the preoperative inflammation response factor showed that there were no significant difference between the two groups in platelet count, neutrophil lymphocyte ratio and platelet lymphocyte ratio. Other examination like total bilirubin, albumin and ALT were also found no significant difference between the two groups. Result of multivariate analysis with logistic regression analysis are summarized in Table 3. It shows that tumor size larger than 3.5 cm (OR=2.205, 95%CI: 1.123- 4.333, P=02) and poorly differentiated tumor cell (OR=1.470, 95%CI: 1.040-2.077, P= .03) were independent risk factor of MVI in non-metastasis single tumor HCC. Other risk factor such as tumor margin especially unsmooth tumor margin and AFP level ≥100 μg/L are not statistically significant in the multivariate analysis.
Table 2: Univariate analysis of clinical characteristic according to microvascular invasion.
Note: MVI: Microvascular Invasion; Hbs-Ag: Hepatitis B Virus Surface Antigen; AFP: Alfa-Fetoprotein; CEA: Carcinoembryonic Antigen; NLR: Neutrophil Lymphocyte Ratio; PLR: Platelet Lymphocyte Ratio; ALT: Alanine Aminotransferase.
Table 3: Multivariate logistic analysis of clinical characteristic according to microvascular invasion.
Note: AFP: Alfa-Fetoprotein.
Discussion
Previous study shown that presence of MVI is one of the most important risk factor related to postoperative tumor recurrence and a prognostic factor associated with lower survival rate [13-16]. This make prediction of MVI become important for surgeon to make a good planning treatment for HCC patients. Therefore, this study was designed to investigate the risk factor of the microvascular invasion in HCC patient to help predict the MVI. In our results, tumor differentiation and tumor size are both significant in univariate and multivariate analysis, those these two variables are the independent risk factor of MVI in non-metastasis single lesion HCC. Poorly differentiated tumor has a higher risk than the other differentiation tumor (OR=1.470, 95%CI: 1.040- 2.077, P= .03). Some published studies found the presence of MVI is closely related to tumor histological differentiation [18]. Du et al. [19] found that tumor histological grade is a strong predictor of MVI and also an important prognostic factor for survival of Small Hepatocellular Carcinoma (SHCC) patients. Esnaola et al. [20] also said that patient with poorly differentiated or undifferentiated tumors were 6 times more likely to have MVI than those with well differentiated tumor. However, even tumor differentiation is an independent risk factor of MVI, the current data of tumor differentiation was taken from histology examination after surgical procedure. So it is hard to make this variable to be a predictor of MVI before the surgery. Pawlik et al. [21] demonstrate that needle core biopsy of HCC to determine histologic grade was inaccurate due to heterogenous tumor differentiation resulting in sampling error. In addition, there is a risk of needle-tract implantation as a complication of biopsy [22]. Therefore, to found another way to differentiate tumor cell before the surgery could help it to become an important predictor variable for the MVI.
Most studies found that tumor size is an independent predictor of MVI in HCC patient, Esnaola et al. [20] found that patient with tumor larger than 4 cm were 3 times more likely to have MVI than those with tumor diameter 4 cm or less. Yamashita et al. [23] from their study of MVI in SHCC patients also found that tumor diameter larger than 2 cm could be a predictor marker of MVI. Our study also parallel with those previous studies, we found that tumor size larger than 3.5 cm is an independent risk factor of MVI in non- metastasis single lesion HCC (OR=2.205, 95%CI: 1.123-4.333, P= .02). Other than tumor size, we also look at tumor margin in patient’s imaging examination with conventional enhance CT and MRI. The liver cancer study group in Japan made a gross classification scheme for resected HCC in 1997 [24], it has been widely used in Japan. They classified tumor gross type into simple nodular type, simple nodular type with extra nodular growth and multinodular type. However, we divide it in simple subclass of tumor margin as smoot margin tumor and non-smooth margin tumor. We found non-smooth margin tumor has a correlation with MVI in HCC patient, even in multivariate analysis there was no significant result found. Some present studies also try to predict MVI with imaging modalities, modalities like Contrast Enhanced Ultrasonography (CEUS), Contrast Enhanced Computed Tomography (CECT), gadoliniumethoxybenzyl- diethylenetriamine penta-acetic acid enhanced MRI (Gd-EOB-DTPA-MRI) and Flourine-18 fluorodeoxyglucose positron emission tomography (18F- FDG PET/CT) reported to be an informative imaging modality to predict MVI [25]. Moreover, MRI and 18F-FDG PET/CT be the promising modality to predict the MVI in preoperative patient [26-28].
In our univariate analysis we also found that there were significant difference between MVI (+) and MVI (-) group in AFP ≥ 100 μg/L. However, in multivariate analysis the result of this variable was not significant. A lot of study report that tumor marker like AFP have a close correlation in predicting MVI in HCC, with various cut off level are reported and the best cut off value are still unknown [23,29]. That makes this biomarker in predicting MVI still controversial. Some previous study found AFP not only a good predictor of MVI but also an independent predictor for survival rate in HCC with MVI [19]. Still need further investigation about correlation of AFP with MVI in HCC patients. There are some limitation in this research field. First, this study is a retrospective design with limited number of patient. Second, viral hepatitis was the majority (183/210) of patients underlying cause of liver disease in this study. When HCC develops in patients with alcoholic complication as the underlying cause, HCC may have different clinical course. Further studies with larger data to found the best predictor of the MVI in HCC patient before the surgery are needed.
Conclusions
According to the study, we found that it is still possible to predict MVI in HCC before the surgical treatment. We found that poorly differentiated tumor and tumor size ≥3.5 cm are independent risk factor of MVI and could help us to predict the possibility of MVI in non-metastasis single lesion HCC patients. Tumor size could be examine before the surgical procedure with some imaging modality like CT scan or MRI. However, the definitive way to find tumor differentiation is only from histological finding, it is hard to determine it before the surgical procedure. With this few possible factors from this study, we hope it could be a trigger for another research to found a new biomarker or another method to predict MVI in HCC accurately.
Conflict of Interest
All the authors declare that there are no conflicts of interest concerning this study.
Acknowledgment
This work was supported by the Zhejiang International Science and Technology Cooperation Project (NO.2016C04003).
Authors’ contributions
J.H.T. designed the study. J.H.T., D.C. collected and processed data. J.H.T., Z.Q. contributed in data analysis and interpretation. J.H.T., A.L.S. contributed in drafting the article. Z.Q., S.Z. contributed Acknowledgments critical revision of the article for important intellectual content. All the authors read and approved the final version of the manuscript.
Ethics Statement
This study was approved by the Institutional Review Board, First Affiliated Hospital, Zhejiang University School of Medicine, under the guidelines of the Ethics Committee of the hospital, and the Declaration of Helsinki.
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer 136(5):E359-E386.
- Fattovich G, Stroffolini T, Zagni I, Donato F (2004) Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 127(5 Suppl 1):S35-S50.
- (2010) Prevention of hepatocellular carcinoma in the Asia-Pacific region: Consensus statements. Journal of gastroenterology and hepatology 25(4):657-663.
- Janevska D,Chaloska-Ivanova V, Janevski V (2015) Hepatocellular Carcinoma: Risk Factors, Diagnosis and Treatment. Open Access Maced J Med Sci3(4):732-736.
- Shimoda M, Tago K, Kato M, Aoki T, Kubota K (2017) Prognostic Factors after Curative Resection for Single-Lesion Hepatocellular Carcinoma in Patients with Normal Liver Function: A Historical Cohort Study. Digestive surgery34(4):281-288.
- Kao WY, Chiou YY, Hung HH, Su CW, Chou YH, et al. (2012) Younger hepatocellular carcinoma patients have better prognosis after percutaneous radiofrequency ablation therapy. Journal of clinical gastroenterology46(1):62-70.
- Hung HH, Lei HJ, Chau GY, Su CW, Hsia CY, et al. (2013) Milan criteria, multi-nodularity, and microvascular invasion predict the recurrence patterns of hepatocellular carcinoma after resection. J Gastrointest Surg17(4):702-711.
- Unal E, Idilman IS, Akata D, Ozmen MN, Karcaaltincaba M (2016) Microvascular invasion in hepatocellular carcinoma. Diagn IntervRadiol22(2):125-132.
- Kao WY, Chiou YY, Hung HH, Su CW, Chou YH, et al. (2012) Serum alpha-fetoprotein response can predict prognosis in hepatocellular carcinoma patients undergoing radiofrequency ablation therapy. Clinical radiology67(5):429-436.
- Uchino K, Tateishi R, Shiina S, Kanda M, Masuzaki R, et al. (2011) Hepatocellular carcinoma with extrahepatic metastasis: Clinical features and prognostic factors. Cancer117(19):4475-4483.
- Gouw AS, Balabaud C, Kusano H, Todo S, Ichida T, et al. (2011) Markers for microvascular invasion in hepatocellular carcinoma: Where do we stand? Liver Transpl17 Suppl 2:S72-S80.
- Costentin CE, Ferrone CR, Arellano RS, Ganguli S, Hong TS, et al. (2017) Hepatocellular Carcinoma with Macrovascular Invasion: Defining the Optimal Treatment Strategy. Liver cancer6(4):360-374.
- Burak KW (2011) Prognosis in the early stages of hepatocellular carcinoma: Predicting outcomes and properly selecting patients for curative options. Canadian journal of gastroenterology 25(9):482-484.
- Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, et al. (2009) A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology137(3):850-855.
- Zhao H, Hua Y, Lu Z, Gu S, Zhu L, et al. (2017) Prognostic value and preoperative predictors of microvascular invasion in solitary hepatocellular carcinoma ≤ 5 cm without macrovascular invasion. Oncotarget8(37):61203-61214.
- Zhao H, Chen C, Fu X, Yan X, Jia W, et al. (2016) Prognostic value of a novel risk classification of microvascular invasion in patients with hepatocellular carcinoma after resection. Oncotarget8(3):5474-5486.
- Lim KC, Chow PK, Allen JC, Chia GS, Lim M, et al. (2011) Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg254(1):108-113.
- Fukuda S, Itamoto T, Nakahara H, Kohashi T, Ohdan H, et al. (2005) Clinicopathologic features and prognostic factors of resected solitary small-sized hepatocellular carcinoma. Hepato-gastroenterology 52(64):1163-1167.
- Du M, Chen L, Zhao J, Tian F, Zeng H, et al. (2014) Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer14:38.
- Esnaola NF, Lauwers GY, Mirza NQ, Nagorney DM, Doherty D, et al. (2002) Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg6(2): 224-232.
- Pawlik TM, Gleisner AL, Anders RA, Assumpcao L, Maley W, et al. (2007) Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: implications for transplant eligibility. Ann Surg245(3):435-442.
- Sparchez Z, Mocan T (2018) Contemporary role of liver biopsy in hepatocellular carcinoma. World journal of hepatology10(7):452-461.
- Yamashita YI, Imai K, Yusa T, Nakao Y, Kitano Y, et al. (2018) Microvascular invasion of single small hepatocellular carcinoma </=3 cm: Predictors and optimal treatments. Ann Gastroenterol Surg 2(3):197-203.
- (2010) Japan LCSGo. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer. 3rd Tokyo: Kanehara & Co., Ltd.
- Tamai H (2018) The prediction of microvascular invasion of hepatocellular carcinoma using multiple imaging modalities. Hematoma Research4:75.
- Cuccurullo V, Di Stasio GD, Mazzarella G, Cascini GL (2018) Microvascular Invasion in HCC: The Molecular Imaging Perspective. Contrast Media Mol Imaging2018:9487938.
- Chou CT, Chen RC, Lin WC, Ko CJ, Chen CB, et al. (2014) Prediction of Microvascular Invasion of Hepatocellular Carcinoma: Preoperative CT and Histopathologic Correlation. American Journal of Roentgenology 203(3):W253-W259.
- Zhang Y, Yang W, Ren J (2018) Risk factors of microvascular invasion in patients with hepatocellular carcinoma. Biomedical Research29(4):697-701.

 Research Article
Research Article