Abstract
Apnea of Prematurity (AOP), defined as cessation of breathing lasting for more than 15 seconds and accompanied by hypoxia and/or brady cardia, is a serious risk factor for premature newborns. India has 3.5 million premature babies who are at risk of apneas every year. Prolonged apnea associated with hypoxia and bradycardia may contribute to hypoxic-ischemic injury of the immature brain. Research till date shows that higher frequency and severity of AOP are associated with a higher incidence of adverse outcomes or death, especially in low income settings. AOP is resolved by physical stimulation, often in the form of manual flicking of the baby’s sole which triggers a nerve response to stimulate respiratory activity. Our innovation, ApneBoot, is designed to give an assured and instantaneous stimulation in response to an apnea by automating the standard of care flicking process. It alerts for bradycardia, hypoxia (using a built-in pulse oximeter) and resolves episodes of central apeas in newborns by instantaneous tactile stimulation to the foot sole. Devices currently available for managing neonatal apnea, are expensive; imported from high income countries; and only sound an alarm if no breathing is detected but do correct the apnea, making ApneBoot a novel device in the market. The pilot study for ApneBoot reports its accuracy as 94% for apnea detection and 70% for apnea resolution. Ongoing and future studies will look at the efficiency of ApneBoot in reducing the harmful consequences of prolonged hypoxia and also observe if it helps in reducing the workload of hospital personnel.
Abbreviations:AOP: Apnea of Prematurity; PCM: Protection Circuit Module; DPPM: Dynamic Power Path Management; BD: Bradycardia and Desaturation; SNCU: Sick Newborn Care Unit; USAID: United States Agency for International Development;
Introduction
India, each year 3.5 million babies are born premature
ranking it first among the top 10 countries with the greatest number
of preterm births. Amongst these, approximately 361,600 children
under five die due to direct preterm complications one of which is
the Apnea of prematurity (AOP) [1]. Apnea of Prematurity (AOP) is
defined as a ‘cessation of breathing by the neonate for more than 15
seconds with hypoxia and/or bradycardia’ [2]. Unresolved apneas
are associated with hypoxia leading to other complications such
as hypoxic-ischemic injury of the immature brain, impaired neuro
development and even death [3]. Almost all infants born at <29
weeks gestation or <1,000 g, 54% at 30 to 31 weeks, 15% at 32 to
33 weeks and 7% at 34 to 35 weeks gestation exhibit apnea, making
the burden of AOP substantial [4]. AOP occurs in part due to poor
nervous system coordination and physical stimulation, often in the
form of manual flicking of the baby’s sole, triggers a nerve response
[5]. This excitatory nonspecific neuronal activity in the brainstem
centre stimulates respiratory activity in the newborn. However, it
is interesting to note that these stimulations only resolve central
apneas and not obstructive apneas. Several studies have examined
the therapeutic effects of mechanical stimulation [6] and found that
tactile stimulations (manual or mechanical) shorten the duration
of hypoxia, bradycardia and in most cases even prevents an apnea.
In low-resource settings, rapid intervention for apnea is
difficult due to lack of vital sign monitors and skilled staff (≈50%
Indian SNCUs have inadequate nursing staff) [7]. These unresolved
apneas may result in injury or death due to prolonged hypoxia. During informal visits conducted by BEMPU in several government
hospitals across India, we observed that the lack of skilled staff
and low nurse to patient ratio leads to sub-optimal management
of apnea in newborns. These findings suggested a need for a device
that not only detects and alerts but also resolves AOP in newborns
in a timely manner. This auto-stimulation device may help in
preventing apnea or hypoxia related injury or death in premature
babies.
ApneBoot: Device Description
ApneBoot is a wearable device that alerts for bradycardia and
hypoxia which are key symptoms of neonatal apnea in premature
newborns. Further ApneBoot also resolves these alerted apnea
episodes and prolonged hypoxia, which is done via an instantaneous
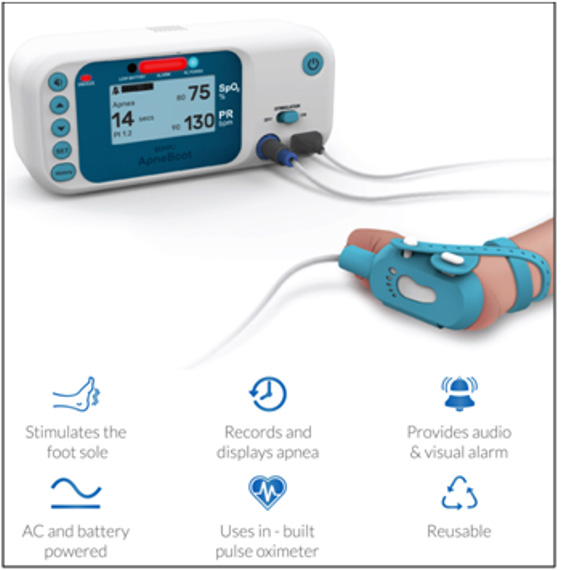
tactile stimulation to the foot sole. The ApneBoot device is designed
with a built-in pulse oximeter (with an alarm) and auto-stimulation
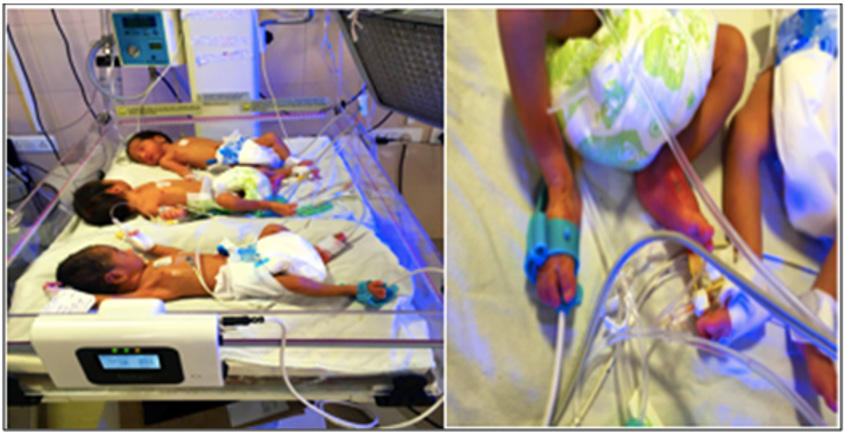
mechanism that fits on the newborns foot. The device can be worn
immediately upon birth and is designed to fit very small babies in
the convenient form of a baby boot (Figure 1). The ApneBoot has
an inbuilt algorithm which monitors for drops in oxygen saturation
and/or heart rate. In the event of an episode, ApneBoot will;
1) Provide a vibro-tactile stimulus on the newborn’s foot
sole, which contains significant nerve endings. This stimulates
the nervous system and restarts breathing.
2) It emits an audiovisual alarm to get the nurse’s attention
in case the apnea is secondary and requires interventions more
than stimulation like assisted ventilation for resolution.
The key Features For The Device Include (Figure 2)
1) The device is reconfigurable to suit all newborns and
gives rapid responses: To enhance the product usability and
acceptability, the device has settings that are reconfigurable
according to the doctor’s recommendation. The device can
be customized and used either only for monitoring and/
or stimulation. The stimulation threshold intensity can also
be adjusted based on the newborn’s weight& age, medical
conditions and the hospital preferences. It has been reported
that the alarm sounds are distressing for the babies in the
NICUs [8], necessitating alarm volume level feature in the
present device.
2) It has a high accuracy built-in pulse oximeter that displays
apnea duration, oxygen saturation and heart rate: ApneBoot
has a medically approved Nonin pulse oximeter module proven
to be accurate and safe as per the basic safety and essential
performance of pulse oximeter equipment standard (ISO
80601-2-61:2011).This module has an accuracy of ±3 digits for
measuring SpO2 in 70-100% range and ±3 digits for measuring
Pulse Rate in 18-300 bpm range [9].
3) The device works continuously with electric power supply
and also has a battery backup of 12 hours for long transport
durations or under conditions of unreliable power supply: The
Li-ion battery of 2900 mAh used in ApneBoot is in compliance
with secondary battery safety standard (EN 62133:2013).
It has a feature of a protection circuit module (PCM) to avoid
under-voltage and short circuit. The device features dynamic
power path management (DPPM), which reduces the number
of charge and discharge cycles on the battery, allowing for
proper charge termination. This feature is most useful in lowresource
settings where power failures are common with no
battery back-ups and during transportation.
4) ApneBoot is safe, portable, robust, reusable and affordable
making it an appropriate innovation for low-resource settings:
The ApneBoot is evaluated for essential performance and
basic safety under intended use cases. The risk management
file is developed while designing the product as per EN
ISO14971:2012 and includes design mitigation features. The
device is also tested as per IEC 60601-1-2:2007 3rd edition
to ensure the electromagnetic compatibility of the device. ApneBoot software is written using state machine architecture
to implement complex decision-making algorithms for Apnea
detection and to respond rapidly to resolve Apnea before any
injury can happen. The device is a light weight (~1kg) and easy
to carry and designed keeping in mind the ease of use during
transportation.
5) The device has a unique feature of tracking and displaying
history of past bradycardia and hypoxia events that may help
to identify recurring episodes and underlying causes like
infections.
6) The device provides a vibro-tactile stimulation on the foot
sole to trigger the central nervous system and restart breathing:
This stimulation mechanism is designed in the convenient form
factor (using medical grade material) of a boot and includes
two key mechanical parts i.e the sole-facing stimulating surface
and the foot strap to firmly hold the boot to the foot. These
parts are designed with ergonomics consideration for a firm
grip to the sole while balancing comfort for the fragile skin of
the newborns. For effective stimulation, the sole-facing surface
of the boot was designed to have a smooth raised profile with
multiple small sized bumps to act as concentration points. The
boot strap has two length-adjusting straps (mid-foot strap&
the ankle strap) to hold the sole-facing stimulating surface
firmly against the neonate’s foot sole and prevent any lateral
movement under the effect of vibration.
ApneBoot has an edge over the routine pulse oximeters as it provides an intervention to resolves apneas. The dependency on the availability and prompt action by a nurse using only monitoring devices leaves babies vulnerable to risk of unattended apneas, especially in understaffed low resource settings. To the best of our knowledge, we have not come across a device which is an effective combination of pulse oximeter and apnea resolving devices available in the market. BEMPU’s ApneBoot is specifically designed to meet the needs and overcome apnea correction in understaffed and under-equipped hospitals and ambulances.
Clinical Evidence for ApneBoot
ApneBoot is designed and developed with extensive user research with neonatologists and nurses working with preterm babies in NICU settings, especially of low-resource hospitals. A high quality FDA approved pulse oximeter module (OEM III) manufactured by Nonin Inc, USA is used as the built-in pulse oximeter to measure the SpO2 and HR making the device accurate, effective and safe. The initial prototype device was tested in-house for safety and accuracy. We conducted a small pilot study (with ethical permissions from the Institutional Ethical committee) to understand the need for stimulator boot dimensions and the stimulation intensity for the auto-stimulating boot in ApneBoot. The study recruited newborns (both female and males) ranging from 25 weeks to 36 weeks GA, weight (900 gms to 2000 gms) to gather information on the following foot measurements; instep girth, waist girth, ball girth, ankle girth, heel (big toe length, ball width). This information was utilised to design the perfect boot dimensions for effective stimulation and a comfortable grip. For the stimulation intensity, we initially calculated the adequate range of the auto-stimulation force by quantifying the force applied in stimulating respiration by ‘flicking of sole’ in neonates with apnea by various personnel including the nurses, doctors and controls. The stimulation intensity (from the above calculations), stimulation patterns and the number of cycles were finalized based on primary experiments done on the above recruited newborns.
Based on the findings, the stimulations found to restart breathing in the newborns most effectively and safely were included in the final design. Further, evidence was also collected of ApneBoot’s ability to detect Bradycardia and Desaturation (BD), key symptoms of neonatal apnea and resolve apneas using the auto-stimulation. The final ApneBoot device thus uses an in-built algorithm that identifies BD events for any of 3 different conditions: 1) SpO2<85% AND HR<100bpm for 5 seconds OR 2) SpO2<75% for 5 seconds OR 3) HR<90bpm for 5 seconds. The final version of ApneBoot was thus hypothesised to accurately detect Bradycardia and Desaturation (BD) events related to apneas and further effectively auto-resolve and terminate apneic and hypoxic episodes through its tactile stimulating boot. To validate this hypothesis we conducted a prospective observational study at Niloufer Hospital, Hyderabad. The objectives of the pilot study were; 1) to measure the accuracy of a novel device in detecting Bradycardia and Desaturation (B&D) events and 2) to determine its efficacy in resolving apneas in newborns with comparison to standard monitor (which only detects B&D events and alerts).The study recruited n=31 preterm newborns (<34 weeks of gestation) from the Sick Newborn Care Unit (SNCU), were provided with an ApneBoot device and monitored for a total of 47 days Enrolled newborns wearing an ApneBoot device were also connected to a standard monitor for comparison.
The study reports that ApneBoot positively predicts a B&D
event 94.1% times and falsely alarms 5.9% times as compared to
the standard monitor. Visual confirmation showed 56/67 events
to be positive apneas indicating 83.6% B&D events coincided with
apneas that were accurately picked up by ApneBoot. ApneBoot
resolved 35 out of 50 events, making the device’s efficacy of apnea
resolution 70%. The pilot study indicated Apne Boot’s effectiveness
in detecting and alarming B&D events, which coincides with the
apnea, and resolving it by providing foot stimulation [10]. ApneBoot
not only resolves apneas but also aims to terminate episodes of
prolonged hypoxia which are proven to cause injury to preterm
babies [11]. To further validate this hypothesis another study is ongoing on a larger sample size. The ongoing study is an RCT,
being conducted at 2 large centers 1 public & private hospital, on a
total of 76 babies. The primary objective of the study is to compare
the duration of hypoxia in each baby during the study period.
Among the 40 babies enrolled to date at both the sites, results look
promising. It is observed that the intervention group has lower
mean hypoxia duration compared to that of control group in the
neonate population recruited. Based on the published and ongoing
studies, ApneBoot was found to be accurate at detecting bradycardia
& hypoxia events related to apnea. Further, it was also reported to
safely stimulate a newborn to resolve these apnea episodes.
The next step will be to look into the potential use of ApneBoot
in transport and humanitarian settings due to its reusability and
portability. Our future study is being designed to observe and
report feedback from users (nurses and doctors) of the device. We
also aim to quantify the reduction in workload of SNCU nurses for
apnea management before and after ApneBoot use.
Impact to Date & Future Plans
ApneBoot’s primary beneficiaries are the subset of 15 million premature newborns in India and globally receiving care in lowresource settings like the Special Newborn Care Units (SNCUs) in government hospitals as well as Primary Health Centres and Community Health Centers. The publication and ongoing studies on the device has generated visibility and appeal in Government Medical Colleges and private hospitals all over India. To date 25 hospitals all over India reaching over 200 preterm newborns (~80% from lower socioeconomic population) have used the device. One government trial at Calicut Medical College has resulted in a NHM recommendation letter for ApneBoot use. BEMPU plans to monitor future deployments of the device as part of an impact and feasibility evaluation to collect evidence that can be used to drive further adoption.
Conclusion
The need for a device that helps in managing Apnea of Prematurity (AOP) was realized to reduce the burden of morbidity and mortality in the infant population. ApneBoot is a novel device in the market that not only detects and alerts for apnea but also resolves the episodes by tactile auto-stimulation. A recent clinical study reports the accuracy of the device to be 70%, demonstrating its potential in apnea management especially in low resource settings.
Conflict of Interest Statement
No potential conflict of interest is reported by the authors. The authors alone are responsible for the content and writing of the paper.
Financial Support Statement
ApneBoot received a SEED grant from Saving Lives at Birth program - A partnership between the United States Agency for International Development (USAID), the Norwegian Ministry of Foreign Affairs, the Bill & Melinda Gates Foundation, Grand Challenges Canada, the Department for International Development, United Kingdom of Great Britain and Northern Ireland (DFID), and the Korea International Cooperation Agency (KOICA)
Acknowledgment
We sincerely thank Dr. Saudhamini Nesargi, St. John’s Hospital, Bangalore; Dr. Himabindu Singh, Dr. Alimelu Rao, Dr. Swapna Lingaldinna, Nilofer Hospital, Hyderabad for all their valuable clinical inputs. We would like to acknowledge the nursing officer’s from St. John’s hospital, Bangalore and Nilofer Hospital, Hyderabad for their incredible support through the development of the device. We also appreciate the contribution of Dr. Vinita V. Khot, Bempu Health, Bangalore, towards the writing of this paper.
References
- Blencowe H, Simon C, Oestergaard M, Chou D, Moller A, et al. (2012) National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. The lancet 379(9832): 2162-2172.
- Barrington K, Finer N (1991) The natural history of the appearance of apnea of prematurity. Pediatric research 29(4): 372-375.
- Eichenwald C (2016) Apnea of prematurity. Pediatrics 137(1): e20153757.
- Fairchild K, Mohr M, Paget Brown A, Tabacaru C, Lake D, et al. (2016) Clinical associations of immature breathing in preterm infants: Part 1-central apnea. Pediatric research 80(1): 21-27.
- Zhao J, Gonzalez F, Mu D (2011) Apnea of prematurity: From cause to treatment. European journal of pediatrics 170(9): 1097-1105.
- Cramer JE, Dekker J, Dankelman J, Pauws SC, Hooper SB, et al. (2018) Effect of tactile stimulation on termination and prevention of apnea of prematurity: A systematic review. Frontiers in pediatrics 6: 45.
- Neogi SB, Khanna R, Chauhan M, Sharma J, Gupta G, et al. (2016) Inpatient care of small and sick newborns in healthcare facilities. Journal of Perinatology 36(3): S18-S23.
- McClure C, Jang SY, Fairchild K (2016) Alarms, oxygen saturations, and SpO2 averaging time in the NICU. Journal of neonatal perinatal medicine 9(4): 357-362.
- https://www.nonin.com/support/oem3
- Lingaldinna S, Singh H, Sharma M (2020) Efficacy of a novel device to detect, alert and resolve neonatal apnea- Pilot study. Innovative Journal of Medical & Health Sciences.
- Poets Christian F, Roberts RS, Schmidt B, Whyte RK, Asztalos EV, et al. (2015) Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. Jama 314(6): 595-603.

 Review Article
Review Article