Abstract
Background: Aerosol administration of medication to animals is much more complicated than in humans due to particle size in relationship to animal size, breathing patterns and lack of cooperation.
Methods: Preliminary in vivo work suggested that using the nebulizing flow to ventilate the animal with an increased tidal volume would enhance both pulmonary deposition and distribution. This was tested using a breath simulator coupled to a modified Aero Eclipse II Breath Actuated Nebulizer™ to evaluate the output captured on a filter at the end of a 3.5 mm endotracheal tube. Two respiratory patterns were used, infant and child. Timed pulses of 50 psi (flow 8 L/m) were applied to the nebulizer in order to achieve a “transpulmonary pressure” of 15 to 20 cmH2O during nebulization. The nebulizer was charged with 5 mL of a solution of albuterol.
Result: The infant breathing pattern yielded a mean of 30% of the nebulizer charge on the inspiratory filter with a mean tidal volume of 100 mL whereas for the child pattern the yield was 46% with a mean tidal volume of 287 mL.
Conclusion: These results compare very favourably with other systems proposed for aerosol delivery to animals.
Introduction
Testing medication on animals is a key final step before
beginning testing in humans. Because of genetic similarity, nonhuman
primate (NHP) is the preferred animal for both efficacy and
toxicology testing in genetic replacement therapeutic programs
such as lung gene therapy for cystic fibrosis. For parental delivery,
dosing is simple in that all the medication is delivered. For enteral
medication, bioavailability comes into play but can usually be
predicted from the dose administered and the blood level achieved.
However, when targeting pulmonary delivery for an agent expected
to stay largely in the lungs, predictable dosing can be a challenge.
One option of direct delivery to the lungs was the Penn Century
Microsprayer [1,2] which is no longer made or available. Another
option has been the Aero Probe™ made by Trudell Medical
International (also currently not commercially available) which has
shown promise in the airway delivery of lung gene therapy vectors
in an intubated rabbit model [3]. This multilumen catheter can be
centered in the endotracheal tube of a deeply anesthetized animal
which can be ventilated by the pulses of air from the controller
which nebulizes a liquid extruded from the center lumen. The
ensuing droplets are in the order of 8-10 μm in diameter and have
been shown to effectively transfer genetic material to the airway [3].
However, droplets of this size may be too large to penetrate deeply
and if alveolar delivery is desired, alternatives may be necessary.
Various nebulizing systems have been proposed for both
spontaneously breathing animals [4] using a face mask and for
intubated animals. MacLoughlin and colleagues [4] duplicated
breathing patterns of NHPs and them mimicked this pattern with
a breath simulator. In the in vitro set up using a vibrating mesh nebulizer on a t piece there was no endotracheal tube or face mask
connection between the delivery device and the “inspiratory” filter
and they estimated that they would delivery a mean of 32% of the
charge dose (range 24-48%) Particle size was not considered and
any material arriving on the “inspiratory” filter was considered as
delivered to the lungs. Unfortunately, in other studies, the delivery
efficiency is relatively low, in the order of 2%, and variable [1].
While this is not a problem for inexpensive drugs, it can be a serious
drawback for expensive hard to make products such as those used
for lung gene therapy. Furthermore, dose ranging studies require
a reasonable degree of predictability of delivery from the device
utilized and variability can be considerable in spontaneously
breathing animals [2]. The purpose of this communication is to
describe an efficient and predictable delivery system for use in
intubated non-human primates (NHP) as a prelude to preclinical
studies in a lung gene therapy program for cystic fibrosis.
Materials and Methods
The nebulizer chosen was the Aero Eclipse™ II Breath Actuated Nebulizer (AE II BAN) made by Trudell Medical International (London, Ontario). Based on the previous experience ventilating rabbits via the controller for the Aeroprobe, it was decided to ventilate the NHPs from the flow from the AE II BAN driven by the original controller for the AeroProbe, the LABneb™CCU (CCU), which was available from previous studies although is also not currently commercially available. This is a 50-psi source with a flow of approximately 8 L/min through the nebulizer. The CCU can be programmed to deliver pulses of varying duration which controls the inspired volume. The AE II BAN is connected to a t-piece that leads directly to the connections for a 3.5 mm endotracheal tube (ET). The right angle of the “t is directed upwards and connected directly onto the expiratory filter and to another t-piece (Figure 1). One limb of this goes to a manometer (and a 20 cm H2O pop off valve in the in vivo set up) while the other is open during expiration and closed during inspiration. The circuit can be occluded manually during the pulses and the duration set by the size of the NHP in order to deliver a transpulmonary pressure between 15 and 20 cmH2O. At the end of each pulse the occlusion was released to allow expiration to occur. The AE II BAN is designed to be breath actuated when the subject creates a negative pressure due to inspiratory flow through the device. It is not meant to have back pressure and during preliminary testing the “inspiratory” valve leaked at the targeted transpulmonary pressures which resulted in losses of gas and aerosol at the top. Despite this, nebulization of methylene blue dye demonstrated widespread and deep penetration to the lungs of an NHP (All California and US Animal Ethics Requirement were met). In order to reduce these leaks, the inhalation port was sealed by gluing the valve with a cyanoacrylate adhesive. To provide a more consistent pressure profile, the edges of the diaphragm were sealed with the adhesive as well.
Figure 1: Figure shows the experimental set up. The components are numbered and described in detail below.
1. AeroEclipse II Breath Actuated Nebulizer™ (Trudell Medical International).
2. Connecting t-piece.
3. Connector to Endotracheal Tube.
4. 3.5mm cuffed Endotracheal tube.
5. Connector between endotracheal tube and inspiratory filter.
6. Inspiratory Filter.
7. Expiratory Filter and connector.
8. T-piece (Intersurgical connector Part # 18006000) to manometer (not shown) and thumb occlusion port.
9. Lung Simulator.
10. LABneb™Catheter Control Unit (CCU).
For the in vitro set up, an electrostatic filter was placed at the end of the endotracheal tube that would normally have gone into the NHP. In the in vivo setting, the anesthetized NHP is held in the sitting position with the nebulizer above so that any rain out of aerosol tends to run into the lungs by gravity and is preferentially directed to the dependent lobes. In the in vitro setting, some rain out may be lost at the connector to the inspiratory filter although virtually all aerosol that arrives at the filter is captured. Expiration is through another electrostatic filter that is used for quantification of recovery in the in vitro setting but also to prevent environmental contamination from biological active viral vectors in the in vivo setting. The total “dead space” from where the AE II BAN connects to the filter on the inspiratory limb is 74 mL (measured by water displacement) with the inspiratory filter which would not be present in vivo having a dead space of 24 mL. The filter exits into a lung simulator with settings of either “infant” or “child.” The lung simulator was an IngMar Medical ASL 5000 (Pittsburg, PA). Prior to charging the nebulizer, the pulse duration is adjusted to achieve the desired “transpulmonary” pressures. Because the electrostatic filters lose their effectiveness over time, they were changed after a cumulative nebulization time of roughly 120 seconds during the studies. Droplet size characterization was accomplished by laser diffraction using Malvern Spraytec particle size analyzer (Malvern Instruments Ltd Worcestershire, United Kingdom) with RT Sizer software. In order to minimize the potential for droplet evaporation, without fouling the lens of the instrument, the horizontal distance between the distal end of the endotracheal tube and detector lens was maintained at 2 cm. A 5 L/min vacuum flow was applied to collect the emitted aerosol and prevent re-circulation through the measurement beam. Mass median diameter (MMD) is anaverage based on the data acquisition period which occurred when beam obscuration exceeded 3% and continued for 15s.
Studies were done in triplicate using both the infant and child respiratory pattern. The nebulizer was charged with 5 mL of an 833 µg/mL solution of albuterol which is the standard used by Trudell Medical (US albuterol ampules 2.5 mg in 3 mL) The number of pulses varied as the device was run to dryness which was loosely defined as the absence of any visible aerosol for at least 10 consecutive pulses. To ensure the validity of the measurements recovery of the active pharmaceutical ingredient was collected from all components of the delivery system (i.e. inspiratory and expiratory filters, residual mass within in the device and connectors) and reported as a percentage of the initial charge placed with the nebulizer. At dryness, all equipment was disassembled and washed with methanol and quantitatively assayed for albuterol. Twenty mL of methanol was added to all inspiratory, and expiratory filters. The filters were then mixed using a Fisher Scientific Vortex mixer. Samples were taken from each filter and placed in 2 mL HPLC vials for analysis. The connectors and device were washed with 20 mL and 40 mLs of methanol respectively place in 2 mL vials for analysis by HLPC (Agulent , Santa Clara, Ca,) for albuterol. Expected deposition was defined as the sum of the amount of albuterol on the inspiratory filters and recovery was the sum of all albuterol remaining including that in the AE II BAN, the expiratory filter and all tubes and connections between the inspiratory and expiratory filters. Both were expressed as a percent of the initial charge dose.
Result
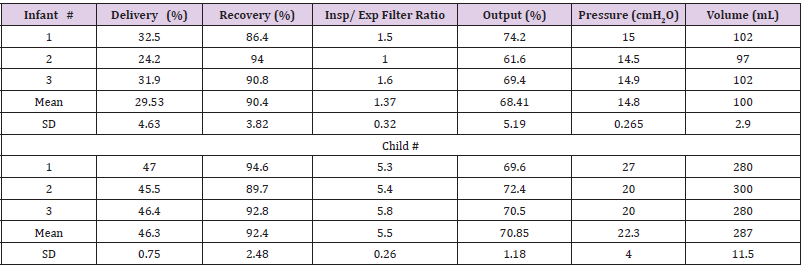
The Table 1shows the individual data points for all experiments. For the infant lung model, the expected (in vitro) lung deposition was 29.5±4.6% of the AE II BAN charge (mean ± one standard deviation (SD)) with a recovery of 90.4±3.8% of the initial charge. The output of the nebulizer was 68.4±5.2 % of the charge. For the child lung model, the expected (in vitro) lung deposition was 46.3.5±0.8% of the AE II BAN charge with a recovery of 92.4±2.5% of the initial charge. The output of the nebulizer was 70.9±1.2 % of the charge. The volume delivered to the lung model for the “infant” was 100±3 mL with a resulting peak pressure of 14.8±2.9 cmH2O. In contrast, for the child model, the volume was 287±12 mL with a peak pressure of 22.9±4.0 cmH2O. This larger volume gave rise to a much higher ratio of albuterol collected on the inspiratory filter compared to the expiratory filter; 5.5±0.26 versus 1.37±0.32 demonstrating the effect of the larger volume delivered for the same apparatus dead space. Individual data are given in the Table 1. The particle size distribution showed a mass median diameter (MMD) of 2.07 µm with geometric standard deviation of 1.57.During dismantling of the second infant run, a drop of nebulisate spilled from the inspiratory filter casing. It was taken up with a blotter but inadvertently added to the connectors rather than to the inspiratory filter assay. This and a slightly lower output for this run resulted in a lower deposition.
Discussion
The results would suggest that there was not only a relatively efficient delivery of the initial charge but also that there was reasonable consistency, The high recovery fraction despite observed small losses at the junction of the endotracheal tube and the inspiratory filter supports the accuracy of the results. The idea of using intermittent positive pressure breathing (IPPB) to increase the delivery of aerosol is not new. Since the 1970’s the Bird Ventilator with a nebulizer in the circuit has been used to deliver medication to patients. What is unique about this set up is that the driving force for the nebulizer and the inspiratory flow is the same which may reduce rain out in the ventilator circuit which would reduce aerosol deposition with the classic Bird Ventilator-IPPB set up.
While the efficiency of this system is less than that of the no longer available Penn Century Microsprayer[1,2], the preliminary study in an NHP showed wide spread distal deposition of dye whereas nuclear medicine studies with the Penn Century Microsprayer suggest very proximal deposition[1]. This diffuse distribution is the result of a very small particle size distribution (MMD 2 μm) coupled with the larger ventilator volumes compared with normal tidal breathing. With normal breathing, the majority of the inspired volume goes to the mid lung zones [5] but an augmented volume would be expected to increase delivery to both upper and lower lung regions. In fact, the estimated delivery is in the order of 10 fold greater than what has been reported for spontaneous tidal breathing with other systems1;2 although, for the infant set up, comparable to that described by MacLoughlan et al [4] but with less variability. Like them, in the current study, increased inspiratory volume meant increased efficiency, as high as 46% in the child lung model in the current study.
There are some limitations to this study. It is recognized that rain out in the ET will end up in the dependent areas of the lungs through gravity, a feature that was obvious in the preliminary NHP study and will contribute to non-uniform aerosol distribution. In the in vitro set up, some of this rain out will end up on the inspiratory filter. The assumption that whatever was captured on the inspiratory filter represents aerosol that would deposit in the lung is not entirely valid. Deposition of rain out in the ET has already been recognized but some of the aerosol that would end up in the large airways in vivo at the end of expiration would be washed out at the start of inspiration but in the in vitro model, this would be captured on the inspiratory filter. However, such losses would be offset by the lower dead space in vivo since the 24 mL of additional dead space from the inspiratory filter would not be there.
The increased ratio of deposition on the inspiratory filter to that on the expiratory filter with larger ventilator volumes means that the effect of apparatus dead space would be greater in smaller animals. Another was the lung model that was used. In this device, expiration is retarded to mimic the normal physiological post inspiration, inspiratory muscle activity which maintains lung volume during expiration to enhance gas exchange. This results in a sinusoidal pattern of both inspiration and expiration where inspiration is shorter than expiration. However, when deeply anesthetized, this pattern is lost with expiration becoming a rapid emptying of the lungs and the expiratory phase becoming shorter [6]. Because of the intent to ventilate the NHP with the nebulizer delivery system, the level of anesthesia necessary to prevent the animal from fighting the “ventilator” was deep and maintained and expiration was much more rapid that that seen in the in vitro model used. The biggest issue with this is that it greatly increased the time required to go to “dryness” because it required an artificial expiratory pause. This was not appreciated initially and failure to allow for complete expiration of the first “child” model led to a recording of a higher peak pressure than that seen later when a greater pause was used. In summary, this experimental aerosol delivery system designed for animals in the 3 to 10-kilogram range would appear to be more efficient than other systems described. The use with larger animals may be possible but the inspiratory flow is limited at 133 mL/second so there would be a limit as to the volume that could be achieved in a reasonable inspiratory time. The particle size distribution is such that widespread peripheral deposition would be anticipated. The system is relatively simple and could provide a valuable resource to investigators should it become available for more widespread use.
Author Disclosure Statement
Dr Coates has done non remunerated consulting work with 4D Molecular Therapeutics and has done collaborative research with Trudell Medical International. The study was sponsored by 4D Molecular Therapeutics and Trudell Medical International.
Author Contribution
Drs Coates and Johnson developed the concept based on a successful preliminary in vivo study. Ms Doyle developed the interface between the nebulizer and associated connectors and the breath simulator as well as doing the HPLC analysis. Mr Coultes was responsible for modifying the nebulizer to prevent aerosol leaks when pressurized. Mr Nagel oversaw all laboratory work and made the particle size measurements. All authors assisted in the preparation of the manuscript.
References
- Beck SE, BL Laube, CI. Barberena AC, Fischer AJ, Adams K Chesnut TR Flotte, WB Guggino. (2002) Deposition and Expression of Aerosolized rAAV Vectors in the Lungs of Rhesus Macaques. Molecular Therapy 6: 546-554.
- Guggino WB, BJ Seagrave, YZ Engelhardt, GG Conlon, L Cebotaru (2017) A Preclinical Study in Rhesus Macaques for Cystic Fibrosis to Assess Gene Transfer and Transduction by AAV1 and AAV5 with a Dual-Luciferase Reporter System. Hum Gene Ther Clin Dev 28: 145-156.
- Koehler DR, H Frndova, K Leung, E Louca, D Palmer, et al. (2005) Aerosol delivery of an enhanced helper-dependent adenovirusformulation to rabbit lung using an intratracheal catheter. J Gene Med 7: 1409-1420.
- MacLoughlin RJ, G van Amerongen, JB Fink, HM Janssens, P Duprex, et al. (2016) Optimization and Dose Estimation of Aerosol Delivery to Non-Human Primates. J Aerosol Med PulmDrug Del. 29: 281-287.
- West JB (1997) Inequality of blood flow and ventilation in the normal lung. Ventilation/Blood flow and gas exchange,(3rd,). Blackwell Scientific Publications, Oxford London Edinburgh Melborne: 15-31.
- Meakin G, AL Coates (1983) An evaluation of rebreathing with the Bain circuit system during anesthesia with spontaneous respiration. Br J Anaesth 55: 487-496.

 Research Article
Research Article