Abstract
Background: Thelevelofsunultravioletrayreachingthesurfaceoftheearthis increasing severely due to the rapid development of the society andenvironmental destruction.Excessiveexposuretoultravioletradiationcausesskindamageand photoaging.Therefore,itisemergedtodevelopeffectivesunscreentoprevent ultraviolet-induced skindamage.
Objective: ThisstudywasaimedtoinvestigatetheeffectsofCoenzymeQ10(CoQ10) sunscreen on the prevention of ultraviolet B radiation (UVB)-induced skindamage.
Methods: 3-month-old female mice were used and randomly divided into fourgroups:control, model, CoQ10, and titanium dioxide (TiO2; positive control) groups. The control mice were not subjected to any treatment, and the other mice were administered with blank (model) or standardized sunscreen (CoQ10orTiO2) for 8 weeks and exposed to UVB light for 30 mins Biochemicalassays, histomorphology, and measurement of matrix metalloproteinase-1 (MMP-1) andDNA (cytosine-5)-methyltransferase 1 (DNMT1) levels were performed in skintissues.
Results: Our results showed that body weight, superoxide dismutase (SOD)and glutathione peroxidase (GSH-Px) activities, and DNMT1 protein expressionwere significantly decreased, while malondialdehyde (MDA) activity and MMP-1level were increased in UVB-treated mice. Besides,the stratum corneum was shed in the model group compared with control group. Incontrast, CoQ10sunscreen prevented UVB-induced skin damage, as well as reversing SOD,GSH-Px and MDA activities, MMP-1 and DNMT1 levels.
Conclusion: The current study provided further evidences on the preventionof UVB-induced skin damage by CoQ10 and its under lying mechanisms.
Keywords: Ultraviolet damage; Coenzyme Q10; Matrix Metalloproteinase; Skin Pathology; Spectroscopy
Abbreviations: CoQ10:Coenzyme Q10;DNMT1: DNA (cytosine-5)-methyltransferase 1; ECM:Extracellular matrix; GSH-Px:Glutathione peroxidase; H&E:Hematoxylin and Eosin; IL:Interleukin; MDA: Malondialdehyde; MMP-1: Metalloproteinase-1; MMPs: Matrix metalloproteases; MPO: Myeloperoxidase; PLA2: Phospholipase A2; ROS: Reactive oxygen species; SD: Standard deviation; SOD: Superoxide dismutase; TiO2: Titanium dioxide; UV: Ultraviolet; UVA: Ultraviolet A radiation; UVB: Ultraviolet B radiation; VG: Van Gieson
Introduction
Skin is the largest barrier protecting from environmental risk factors that can result in skin aging. Skin aging can be categorized into intrinsic and extrinsic responses. The intrinsic skin aging occurs naturally as time passes [1], while extrinsic factors in skin aging are related with infection, water loss, and ultraviolet ray [2]. Eventhough only 5% of ultraviolet B radiation (UVB) light reaches the upper dermis of the skin, it is a key risk factor of extrinsic skin aging that affects dermal fibroblasts and skin microenvironment [3]. Collagen, a major component of extracellular matrix (ECM), is associated with extrinsic skin aging. Many studies have reported that collagen is degraded by matrix metalloproteases (MMPs), including MMP-1, MMP-8, and MMP-13 [4,5]. In particular, MMP-1 is the predominant collagenase in the skin. Since wrinkle formation is evidenced by collagen degradation, the attenuation of MMP-1 activity is an important method for preventing skin aging [6,7]. On the other hand, skin aging is also associated with decreased activity of anti-oxidant enzymes [8].
The system of oxidant and anti-oxidant tends to be balance under normal conditions [9]. However, the levels of reactive oxygen species (ROS) would be produced excessively when the skin was exposed to ultraviolet ray [10,11]. Therefore, the scavenging capacity of the free radicals and the activities of anti-oxidant enzymes, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), were reduced,while the amounts of free radicals, malondialdehyde (MDA), were increased.CoenzymeQ10 (CoQ10, also known as ubiquinone) was found by Crane et al. in 1957 to be in the mitochondria of the beef heart, and broadly distributed in mammalian tissues [12,13]. CoQ10, a necessary factor for healthy body, plays an important role in cardiovascular disorders and aging, including heart failure, hypertension, and endothelial dysfunction [14]. Growing evidences showed that CoQ10 also has a potential role for the prevention and treatment of heart ailments by improving cellular bioenergetics via scavenging free radicals [15]. With regard to ultraviolet A radiation (UVA)-induced skin aging, CoQ10 might be a useful preventive medication against skin photo-aging [16].
Among CoQ10-loaded conventional carriers, ultra-small lipid nanoparticles-CoQ10 exhibited reduced capacity in free radical formation compared with non-nano carrier-treated cells. Therefore ultra-small lipid nanoparticles containing CoQ10 were shown to be suitable to increase the anti-oxidant capacity of the skin [17]. Moreover, CoQ10 significantly reduced the levels of myeloperoxidase (MPO), phospholipase A2 (PLA2) and MDA, while it increased SOD levels in vivo and in vitro [18]. A study found that CoQ10 might rejuvenate wrinkled skin through inhibiting the degradation of dermal fiber components and stimulating the paracrine of dermal fiber via up-regulation of interleukin (IL)-6 and MMP secretion [19]. As systemic delivery of anti-oxidants to the skin is poor, it may be beneficial to penetrate the skin with sufficient amount as topical application [20,21]. The present study explored the preventive effects of CoQ10 sunscreen against skin damage induced by UVB as topical application on mouse skin.
Materials and Methods
Materials
CoQ10 was provided by Runhe Biology Co. (Guangzhou, China), while titanium dioxide (TiO2) was purchased from KemaoChemical Co. (Dongguan, China).Ointment base was made by our laboratory, which contained purified water,petrolatum, Tween 80, cetostearyl alcohol, and did not contain any drug.
Animals and Treatments
This study was carried out according to the Guide for the Care and Use of Laboratory Animals of Guangdong Laboratory Animal Monitoring Institute, the National Laboratory Animal Monitoring Institute of China. All the procedures performed were in accordance with the ethical standards of the Academic Committee on the Ethics of Animal Experiments of Guangdong Medical University. 36 specific pathogen-free female Kunming mice were acclimated to local vivarium conditions (temperature 24-26℃, humidity 67%) and allowed to free access of water and diets containing 1.11% calcium, 0.74% phosphorus. The average weight of mice was about 27.66 g ± 0.56. CoQ10 sunscreen was composed of CoQ10 and ointment base at the concentration of 10 mg/g. TiO2sunscreen was used as a positive control, and composed of TiO2 and ointment base at the concentration of 50 mg/g[18,19]. mice were randomly138 divided into four groups: control group (n=9), aging model group (ointment base without additives; n=9), CoQ10 group (CoQ10 sunscreen; n=9), and TiO2 group (TiO2 sunscreen; n=9). The hair on the back of each mouse was shaved, and 0.5 g of ointment was topically applied to 3 × 3 cm2 of the skin once daily for 8 weeks. Except the control group, the other groups were exposed to ultraviolet B radiation (UVB; 303 nm and 1522.7 μW/cm2) under diffused UV light (Sentry Optronics CORP, Taiwan) for 30 mins everyday
Sample Collection
All the mice were weighed weekly. At the end of the treatment, the mice were sacrificed and blood was collected from the eyeballs. Firstly, the serum was collected from the blood by centrifugation, and it was used for biochemical assays. And then, the dorsal skin, heart, liver, kidney, and brain were isolated, weighed, and normalized by body weight. Finally, the samples of dorsal skin tissues were immediately collected for histological, biochemical, and quantitative real-time PCR analyses.
Biochemical Analysis
The degree of skin damage exposed to UVB could be determined by evaluating the activities of MDA, SOD, and GSH-Px [22]. They are the most frequently used biomarkers of oxidative stress (imbalance between oxidant and anti-oxidant systems) 158 in the skin tissues. MDA levels in the dorsal skin were measured using a MDA detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instructions of the manufacturer. The activities of SOD and GSH-Px in the skin tissues were detected using a commercial kit (Nanjing Jiancheng Bioengineering Institute).
Histological Analysis
Each part of the skin samples (1×1 cm2) was fixed with 4% paraformaldehyde for Van Gieson (VG), and hematoxylin and eosin (H&E) staining. H&E staining was used to assess the skin structure alteration, while VG staining was applied to detect the presence of collagen fibers. All the stained skin specimens were observed and photographed using an optical microscope (Nikon Eclipse Boi, Nokon Corporation, Japan).
Quantitative Real-Time PCR
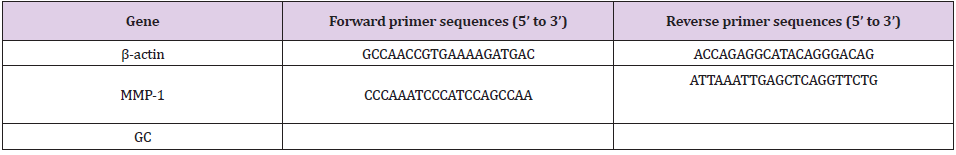
Total RNA was extracted from the dorsal skin tissues using Trizol reagent (TaKaRa Bio, Otsu, Japan) as recommended by the manufacturer. Total RNA was reverse transcribed to cDNA using a commercial kit (Takara Bio, Otsu, Japan) according to the protocol of the manufacturer. Target genes were amplified with SYBR@ Premix Ex TaqTM ǁ (Takara Bio, Otsu, Japan) using a PikoRel 96 Real-Time PCR System (Thermo Fisher Scientific, Vantaa, Finland). The sequences of the forward and reverse primer sets (Shenggong Bio. Co., Shanghai, China) were shown in Table 1. To confirm the specificity of the amplification, PCR products were evaluated by melting curve analysis. mRNA expression was determined based on the cycle threshold values, which were normalized to that of β-actin, and calculated using the 2−ΔΔCT method [23].
Western Blotting
The total protein was extracted from the dorsal skin tissues with ice-cold lysis buffer.The protein concentrations of the lysates were measured by the bicinchoninic acid kit (Pierce, France). Equal amount of proteins were used and separated by SDS-PAGE gels, and were then transferred onto the nitrocellulose membranes. Next, the membranes were incubated with the primary DNMT1 antibody (Cell Signaling, USA), and anti-rabbit-HRP antibody (Cell Signaling, USA). The blots were developed by enhanced chemiluminescence(GE Healthcare Life Sciences, USA) with aChemiDoc™ MP System (Bio- Rad Laboratories, USA). α-tubulin antibody (Cell Signaling, USA) was used as a housekeeping control.
Statistical Analysis
The results were expressed as mean ± standard deviation (SD). The data were analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). The significance of differences between groups was evaluated by one-way or two-way ANOVA, and p<0.05 was considered statistically significant.
Results
CoQ10 Sunscreen slightly Altered Body and Organ Weights in UVB-Treated Mice
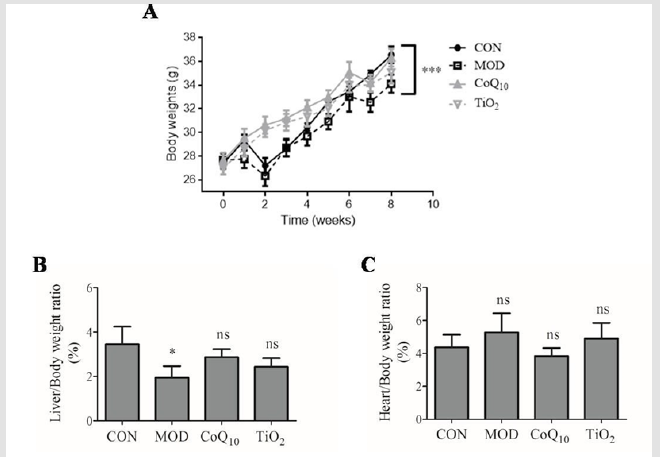
As shown in Figure 1, the body weights of the model mice were significantly decreased compared to control mice. In contrast, the body weights of CoQ10 and TiO2mice were significantly increased compared to model mice. The results also showed that liver weight was significantly decreased when the mice were exposed to UVB compared to control mice (Figure 1). However, there were no statistical differences in other groups. The heart weight was also not affected by CoQ10 and TiO2 sunscreen compared with control mice (Figure. 1).
CoQ10Sunscreen Altered Anti-Oxidant Enzyme Activities in UVB-Treated Skin
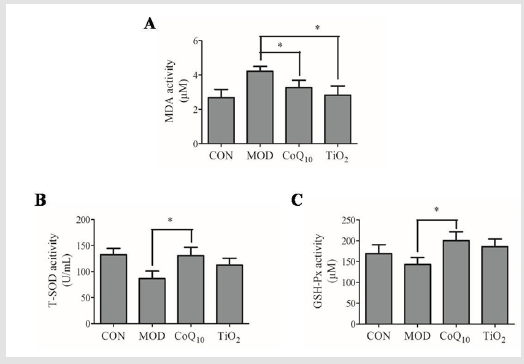
We investigated the activities of anti-oxidant enzymes in UVB-treated skin in response to CoQ10 sunscreen treatment. Compared with control group, the MDA activity was increased in the dorsal skin of the model group (Figure 2). Interestingly, the MDA activity was significantly decreased in CoQ10 and TiO2 groups compared with the model group. Moreover, the activities of T-SOD and GSH-Px were decreased in the dorsal skin of the model group compared with control group (Figure 2).The reductions of these activities were significantly attenuated in CoQ10 group compared to the model group. Similarly, TiO2group, as a positive control, also slightly attenuated these reductions compared to the model group (Figure 2).
CoQ10Sunscreen Protected Epidermis in UVB-Treated Skin
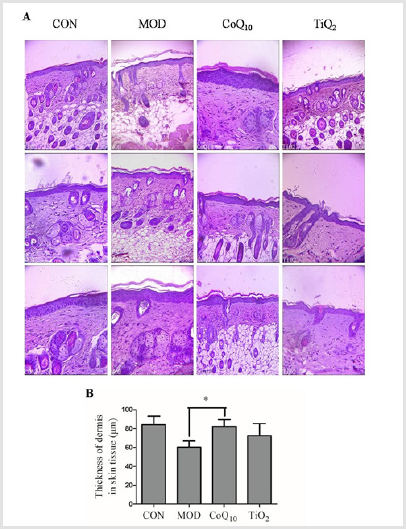
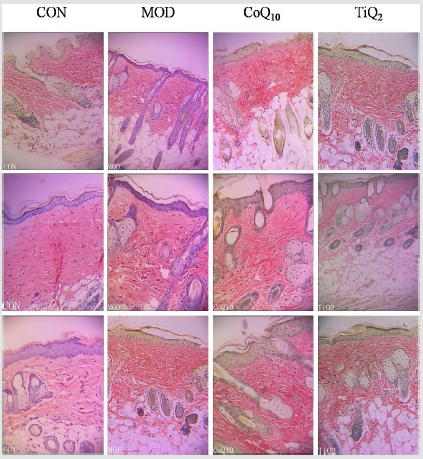
To investigate the effect of CoQ10 sunscreen on the epidermis in UVB-treated skin,H&E staining was used, and the thickness of the dermis was also assessed. Our results showed that the epidermis of control mice was unbroken and its corneum was not shed, but the epidermis of model mice was injured and its corneum was shed obviously (Figure 3). Compared with model group, there were no differences in CoQ10 and TiO2 groups. Interestingly, the skin of CoQ10 mice was not injured and its corneum was not shed, but the skin of TiO2 mice appeared to be a little bit broken. Furthermore, the corneum of TiO2 mice was shed slightly, but it looked healthier than that of model mice. In addition, the thickness of the dermis in the model mice was decreased compared with control mice, and this reduction in thickness was significantly attenuated in CoQ10 mice (Figure 3).
Figure 1: Body and organ weight changes with Coenzyme Q10 (CoQ10) sunscreen treatment in response to ultraviolet B
radiation (UVB). The weights of
a) Body,
b) Liver, and
c) Heart
in response to UVB. CON, control group without exposing to UVB; MOD, model group with ointment base exposed to UVB;
CoQ10, treatment with CoQ10 sunscreen exposed to UVB; TiO2, positive control with titanium dioxide (TiO2) sunscreen
exposed to UVB. n≥3 for each group. Results were shown as mean± SD. *p<0.05 vs. CON, ***p<0.001 CON, CoQ10, TiO2 vs.
MOD.
Figure 2: The anti-oxidant enzyme activities were altered by Coenzyme Q10 (CoQ10) sunscreen treatment in ultraviolet B
radiation (UVB)-treated skin. The activities of
a) Malondialdehyde (MDA),
b) Superoxide dismutase (SOD), and
c) Glutathione peroxidase (GSH-Px)
in the UVB-treated skin tissue in response to CoQ10 sunscreen treatment. CON, control group without exposing to UVB;
MOD, model group with ointment base exposed to UVB; CoQ10, treatment with CoQ10 sunscreen exposed to UVB; TiO2,
positive control with titanium dioxide (TiO2) sunscreen exposed to UVB. n≥3 for each group. Results were shown as
mean ± SD. *p<0.05 vs. MOD.
CoQ10 Sunscreen Prevented Degradation of Collagen in UVB-Treated Skin
In order to investigate the effect of CoQ10 sunscreen on collagen degradation in UVB-treated skin, Van Gieson staining was performed. As shown in Figure 4, we found that the collagen fibers of control mice was deposited neatly, while there were less collagen fibers in model mice they rowed irregularly. Moreover, its corneum was shed obviously in model group. Compared with model group, the skin of CoQ10 mice was not injured and its collagen fibers rowed regularly. However, the skin of TiO2mice appeared to be a little bit broken, its corneum was shed slightly, and its collagen fibers rowed regularly.
Figure 3: Coenzyme Q10 (CoQ10) restored ultraviolet B radiation (UVB)-induced damage in the epidermis of the skin.
a) Hematoxylin and Eosin (H&E) staining of the epidermis and dermis on the skin. The picture was captured at 10×
magnification using an electron microscope.
b) The thickness of the dermis was measured in response to CoQ10 treatment.
CON, control group without exposing to UVB; MOD,model group with ointment base exposed to UVB; CoQ10, treatment
with CoQ10sunscreen exposed to UVB; TiO2, positive control with titanium dioxide (TiO2) sunscreen exposed to UVB.
n≥3. Results were shown as mean ± SD. *p<0.05 vs. MOD.
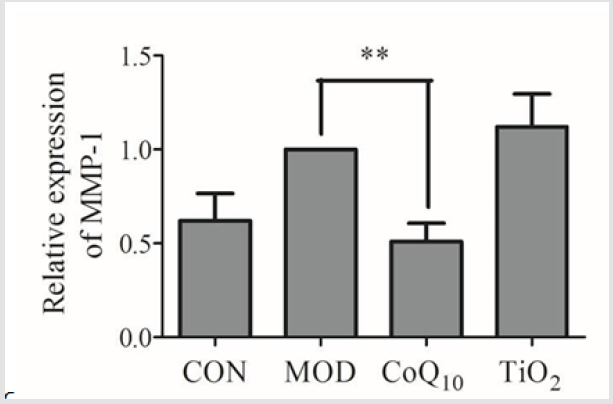
CoQ10 Sunscreen Modulated MMP-1 Expression in UVBTeatedSkin
Skin collagen degradation is mainly regulated by MMP-1 [24]. We found that the mRNA level of MMP-1 of model group was increased compared with control group(Figure 5). CoQ10 treatment significantly attenuated this up-regulation of MMP-1 level induced by UVB. Conversely, the MMP-1 level was not decreased in TiO2 group compared with the model group.
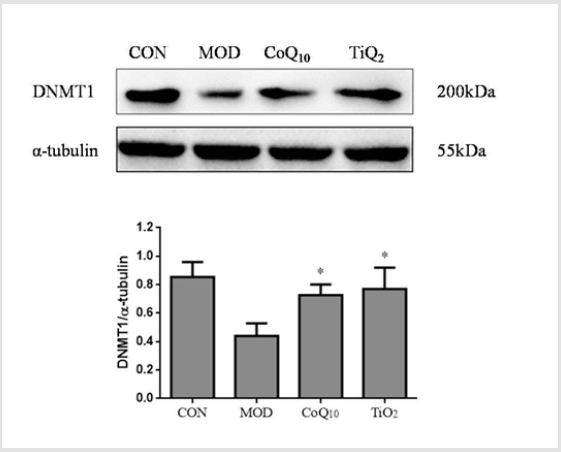
CoQ10 Sunscreen Prevented DNMT1 Down-Regulation in UVB-Treated Skin
DNMT1 was shown to be associated with UV-induced photoaging [25]. Next, we investigated the effect of CoQ10 sunscreen on DNMT1 protein expression in UVB-treated skin. Our results showed that DNMT1 expression was decreased in the model group compared to control group (Figure 6). Besides, CoQ10 and TiO2 sunscreen treatment significantly suppressed this down-regulation induced by UVB.
Discussion
Skin is regarded as the first line of defense against infection and environmental factors such as ultraviolet (UV) radiation. Sunlight is the main source of UV radiation,which can induce skin senescence, inflammation, aging and cancer [26]. Therefore protecting the skin with sunscreen is very important to avoid skin damage. CoQ10 was shown to be an anti-oxidant molecule which could prevent UV-induced DNA damage [19]. In this study, we further investigated the preventive effects of CoQ10 sunscreen on UVB-induced skin damage in mice, and its underlying mechanisms. In this study, we found that mouse skin was damaged by UVB. This was shown by the decrease in growth rate of body weight and liver weight in UVB-treated mice. Besides,UVB decreased the activities of SOD and GSH-Px, and increased MDA activity in the mouse skin. This suggested that the balance between oxidant and anti-oxidant systems was impaired when mouse skin was exposed to UVB only. During the aging process, the skin dermis becomes thin and damaged [27], due to the degradation of the collagen matrix [28,29].
Figure 4: Coenzyme Q10 (CoQ10) sunscreen restored collagen degradation in ultraviolet B radiation (UVB)-treated skin. Van Gieson staining was used to detect collagen on the skin. The picture was captured at 10× magnification using an electron microscope. CON, control group without exposing to UVB; MOD, model group with ointment base exposed to UVB; CoQ10, treatment with CoQ10 sunscreen exposed to UVB; TiO2, positive control with titanium dioxide (TiO2) sunscreen exposed to UVB.
Figure 5: Coenzyme Q10 (CoQ10) sunscreen altered MMP-1 mRNA level in ultraviolet B radiation (UVB)-treated skin. The MMP-1 level of mouse skin was measured by real-time PCR. CON, control group without exposing to UVB; MOD, model groupwith ointment base exposed to UVB; CoQ10, treatment with CoQ10 sunscreen exposedto UVB; TiO2, positive control with titanium dioxide (TiO2) sunscreen exposed to UVB. n≥3. Results were shown as mean ± SD. **p<0.01 vs. MOD
Figure 6: Coenzyme Q10 (CoQ10) sunscreen altered DNMT1 protein expression in ultraviolet B radiation (UVB)-treated skin. Immunoblots and representative graph showing the expression of DNMT1. The expression of DNMT1 was measured by western blotting. CON, control group without exposing to UVB; MOD, model group with ointment base exposed to UVB; CoQ10, treatment with CoQ10 sunscreen exposed to UVB; TiO2, positive control with titanium dioxide (TiO2) sunscreen exposed to UVB. n≥3. Results were shown as mean ± SD. *p<0.05 vs. MOD.
We also showed that the corneum of mouse skin that was exposed to UVB was shed through regulating collagen via up-regulation of MMP-1 expression. Taken together, our mouse UVB model could be a suitable model for skin aging. In the current study, we demonstrated that topical application of CoQ10 sunscreen could alleviate the alterations of collagen in the mouse skin induced by UVB, and the corneum of mouse skin was not shed. MMP-1 expression has been shown to be increased with age, which is a major factor that causes collagen breakdown and wrinkling problems [30,31]. Indeed, aging is the primary consequence of aerobic metabolism, which produces excess ROS and exceeds the capacity of cellular anti-oxidant defense [32]. Therefore, oxidants are important mediators of aging [33]. In fact, ROS production is also related to age-associated up-regulation of MMP-1 [27]. Interestingly, we showed that CoQ10 sunscreen treatment could inhibit MMP-1 up-regulation and collagen degradation induced by UVB in the mouse skin. Similarly, CoQ10 sunscreen was also shown to reduce MMP-1 levels in dermal fibroblasts [19].
Moreover, anti-oxidant enzymes in the skin, including SOD and GSH-Px, can counteract ROS [34]. Our results showed that the activities of SOD and GSH-Px were significantly increased by CoQ10 sunscreen in the mouse skin. Furthermore, MDA is a biomarker of cell membrane damage caused by free radicals [35]. We found that CoQ10 sunscreen could attenuate MDA activity induced by UVB. Taken together, this suggested that CoQ10 sunscreen might have anti-oxidant activities against UVB damage in mouse skin, and prevented collagen degradation by suppressing MDA activity and MMP-1 levels, and enhancing SOD and GSH-Px activities. In summary, our findings indicated that topical application of CoQ10 sunscreen prevented UVB-induced skin damage by enhancing the anti-oxidative capacity against photo-aging skin and delay the breakdown of collagen through suppressing MMP-1 level and MDA activity in the mouse skin. Therefore, we suggested that CoQ10 sunscreen might have beneficial effects in anti-aging, and topical application of CoQ10 sunscreen could be potential protection against UVB-induced photo-aging.
Acknowledgements
We would like to thank Ms. Ziwei Hu and Ms. Jinbing Zheng for their work in animal care. This work was supported by the Joint Center of Guangdong Medical University and Guangdong Run He Biotechnology Company for Co-enzyme Q10 research (2xx14002).
Conflict of interest
The authors declare that there is no competing conflict of interests.
References
- Yaar M, Eller MS, Gilchrest BA (2002) Fifty years of skin aging. The journal of investigative dermatology Symposium proceedings 7(1): 51-58.
- Chung JH (2003) Photoaging in Asians. Photodermatology, photoimmunology & photomedicine19(3): 109-121.
- Bruls WA, van Weelden H, van der Leun JC (1984) Transmission of UV-radiation through human epidermal layers as a factor influencing the minimal erythema dose. Photochemistry and photobiology 39(1): 63-67.
- Pilcher BK, Sudbeck BD, Dumin JA, Welgus HG, Parks WC, et al. (1998) Collagenase-1 and collagen in epidermal repair. Archives of dermatological research 290 Suppl: S37-46.
- Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, et al. (2006) Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. The American journal of pathology 168(6): 1861-1868.
- Fligiel SE, Varani J, Datta SC, Kang S, Fisher GJ, et al. (2003) Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. The Journal of investigative dermatology 120(5): 842-848.
- Xia W, Quan T, Hammerberg C, Voorhees JJ, Fisher GJ, et al. (2015) A mouse model of skin aging: fragmentation of dermal collagen fibrils and reduced fibroblast spreading due to expression of human matrix metalloproteinase-1. Journal of dermatological science 78(1): 79-82.
- Gutteridge JM, Halliwell B (2000) Free radicals and antioxidants in the year 2000. A historical look to the future. Annals of the New York Academy of Sciences 899: 136-147.
- Thiele JJ, Schroeter C, Hsieh SN, Podda M, Packer L, et al. (2001) The antioxidant network of the stratum corneum. Current problems in dermatology 29: 26-42.
- Guyton KZ, Gorospe M, Wang X, Mock YD, Kokkonen GC, et al. (1998) Age-related changes in activation of mitogen-activated protein kinase cascades by oxidative stress. The journal of investigative dermatology Symposium proceedings 3(1): 23-27.
- Harman D (2006) Free radical theory of aging: An update: Increasing the functional life span. Annals of the New York Academy of Sciences 1067: 10-21.
- Prakash S, Sunitha J, Hans M (2010) Role of coenzyme Q(10) as an antioxidant and bioenergizer in periodontal diseases. Indian journal of pharmacology 42(6): 334-337.
- Potgieter M, Pretorius E, Pepper MS (2013) Primary and secondary coenzyme Q10 deficiency: the role of therapeutic supplementation. Nutrition reviews 71(3): 180-188.
- Yang YK, Wang LP, Chen L, Yao XP, Yang KQ, et al. (2015) Coenzyme Q10 treatment of cardiovascular disorders of ageing including heart failure, hypertension and endothelial dysfunction. Clinicachimicaacta; international journal of clinical chemistry 450: 83-89.
- Kumar A, Kaur H, Devi P, Mohan V (2009) Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacology & Therapeutics 124(3): 259-268.
- Tanino Y, Budiyanto A, Ueda M, Nakada A, Nyou WT, et al. (2005) Decrease of antioxidants and the formation of oxidized diacylglycerol in mouse skin caused by UV irradiation. Journal of Dermatological Science Supplement1(2): S21-S28.
- Lohan SB, Bauersachs S, Ahlberg S, Baisaeng N, Keck CM, et al. (2015) Ultra-small lipid nanoparticles promote the penetration of coenzyme Q10 in skin cells and counteract oxidative stress. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnike V89: 201-207.
- Choi BS, Song HS, Kim HR, Park TW, Kim TD, et al. (2009) Effect of coenzyme Q10 on cutaneous healing in skin-incised mice. Archives of pharmacal research 32(6): 907-913.
- Inui M, Ooe M, Fujii K, Matsunaka H, Yoshida M, et al. (2008) Mechanisms of inhibitory effects of CoQ10 on UVB-induced wrinkle formation in vitro and in vivo. BioFactors (Oxford, England) 32(1-4): 237-243.
- Werninghaus K, Meydani M, Bhawan J, Margolis R, Blumberg JB, et al. (1994) Evaluation of the photoprotective effect of oral vitamin E supplementation. Archives of dermatology 130(10): 1257-1261.
- Ahn SM, Hwang JS, Lee SH (2007) Fructose 1,6-diphosphate alleviates UV-induced oxidative skin damage in hairless mice. Biological & pharmaceutical bulletin 30(4): 692-697.
- Hamdan D, El Readi MZ, Tahrani A, Herrmann F, Kaufmann D, et al. (2011) Chemical composition and biological activity of Citrus jambhiri Lush. Food Chem127(2): 394-403.
- Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, et al. (2000) Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Analytical biochemistry 285(2): 194-204.
- Van Doren SR (2015) Matrix metalloproteinase interactions with collagen and elastin. Matrix biology : journal of the International Society for Matrix Biology 44-46: 224-231.
- Yi Y, Xie H, Xiao X, Wang B, Du R, et al. (2018) Ultraviolet A irradiation induces senescence in human dermal fibroblasts by down-regulating DNMT1 via ZEB1. Aging 10(2): 212-228.
- Cleaver JE, Crowley E (2002) UV damage, DNA repair and skin carcinogenesis. Frontiers in bioscience : a journal and virtual library 7: d1024-1043.
- Fisher GJ, Varani J, Voorhees JJ (2008) Looking older: fibroblast collapse and therapeutic implications. Archives of dermatology 144(5): 666-672.
- Fossel M (2002) Cell senescence in human aging and disease. Annals of the New York Academy of Sciences 959: 14-23.
- Bonta M, Daina L, Mutiu G (2013) The process of ageing reflected by histological changes in the skin. Romanian journal of morphology and embryology = Revue roumaine de morphologieetembryologie 54(3 Suppl): 797-804.
- Kumar S, Vinci JM, Millis AJ, Baglioni C (1993) Expression of interleukin-1 alpha and beta in early passage fibroblasts from aging individuals. Experimental gerontology 28(6): 505-513.
- Mawal Dewan M, Lorenzini A, Frisoni L, Zhang H, Cristofalo VJ, et al. (2002) Regulation of collagenase expression during replicative senescence in human fibroblasts by Akt-forkheadsignaling. The Journal of biological chemistry 277(10): 7857-7864.
- Harman D (1992) Free radical theory of Mutation research 275(3-6): 257-266.
- Cutler RG (1991) Antioxidants and aging. The American journal of clinical nutrition. 53(1 Suppl): 373s-79s.
- Truong VL, Bak MJ, Jun M, Kong AN, Ho CT, et al. (2014) Antioxidant defense and hepatoprotection by procyanidins from almond (Prunusamygdalus) Journal of agricultural and food chemistry 62(34): 8668-8678.
- Ayyappan S, Philip S, Bharathy N, Ramesh V, Kumar CN, et al. (2015) Antioxidant status in neonatal jaundice before and after phototherapy. Journal of pharmacy&bioallied sciences 7(Suppl 1): S16-21.

 Research Article
Research Article