Abstract
The recent clinical studies performed to in vivo investigate the immune functions have shown that lymphocyte-to-monocyte ratio (LMR) may be considered as the more simple and synthetic biomarker to clinically analyze the immune status of patients, since the immune activation is mainly related to lymphocyte functions, whereas the monocyte-macrophage system is the main responsible for chronic inflammationrelated immune suppression. Cancer progression has been proven to be associated with a progressive decline in LMR values, while the behavior of LMR in autoimmunity is more controversial. The present preliminary study was planned to analyze LMR values in cancer patients, and in patients with autoimmune diseases in relation to the different disease phases. The study included 149 cancer patients (non-metastatic disease: 68; metastatic disease: 81), 51 patients with autoimmune pathologies (remission phase: 37; acute phase: 14), and in 100 healthy subjects as controls. LMR mean values observed in metastatic patients were significantly lower than those found either in patients without metastases, or in controls. Patients with autoimmune diseases in remission phases showed higher values of LMR than controls, without, however, significant differences, whereas LMR mean values observed in patients during the acute phase of disease were significantly lower with respect to both controls and patients in remission phase. These results seem to suggest that the evidence of abnormally low values of LMR may deserve a negative prognostic significance in both cancer and autoimmunity. This paradoxical evidence may be explained only by investigating the main inflammatory and anti-inflammatory cytokines responsible for the regulation of the immune functions, including TGF-beta, IL-2, IL-17, IL-6, and IL-12, whose detection will be the aim of future clinical studies.
Keywords: Autoimmunity; Cancer; Cytokines; Lymphocyte-to-Monocyte Ratio; Interleukin-2; Transforming growth factor- beta
Introduction
Metastatic cancer and autoimmune diseases are commonly considered to be the expression and the consequence of two opposite immune reactions, consisting of an immunosuppressive status in cancer, and an exaggerated immune activation in the autoimmunity. However, despite the opposite immune behavior occurring in cancer and in autoimmunity, some immune laboratory parameters have appeared to show a negative prognostic significance in both advanced tumors and autoimmune diseases, namely lymphocytopenia itself, and namely the lymphocyte-to-monocyte ratio (LMR) [1-4], which at present represents the most simple and the less expensive biomarker to evaluate the immune status of patients. This paradoxical and apparently controversial evidence may be explained by taking into consideration that lymphocyte count includes different subsets of cells with different activities, as well as by considering the physiology of the cytokine network with its complex positive and negative feed-back mechanisms. According to the more recent advances in the knowledge of the physiology of the immune system, the different immune reactions are substantially the result of the interactions between lymphocyte and macrophage systems, which may be synthetized by the values of LMR.
In fact, lymphocyte count has been proven to be namely correlated to TH1 lymphocytes [5], the main cells responsible for the immune activation, and monocyte number has appeared to reflect the functional status of the macrophage system, including tissue macrophage infiltration [6,7]. Moreover, from an immunological point of view, it is known that metastatic cancers are namely characterized by a progressive decline in TH1 lymphocyte number in association with an increase in regulatory T lymphocyte (T reg) count and activation [5]. Then, cancer-related immune suppression would depend on both diminished immune activation, namely mediated by TH1 cells, and increased immune suppression, induced by T reg cells [8]. On the other side, the autoimmune diseases are mainly characterized by a decline in T reg cell count and activity, in association with an increased TH17 lymphocyte number, with a following enhanced IL-17 secretion [9], while the behavior of TH1 lymphocytes is still controversial, as well as that of total lymphocyte count, and LMR values. The controversial results could simply depend on the fact that the decline in LMR values may depend on different conditions, consisting of a decline in lymphocyte count alone, an increase in monocyte count alone, and a decline in lymphocyte count in association with an increased monocyte count. These different profiles of LMR could reflect different immune pathogenetic behaviors. On these bases, a preliminary study was planned to evaluate LMR values in cancer and autoimmunity in relation to the different phases of the clinical course, by comparing cancer patients with locally limited disease to those with metastatic disease, and patients with autoimmune diseases in remission phase to those with disease exacerbation.
Materials and Methods
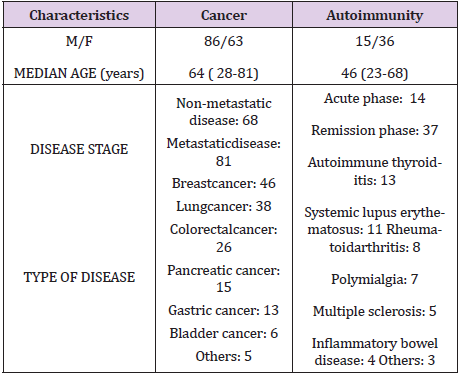
The study included 200 consecutive patients, 149 of whom were affected by solid neoplasms, and the other 51 patients by autoimmune diseases. Moreover, within cancer group, 68 patients showed a locally limited disease, while the other 81 had a metastatic disease. Within autoimmunity group, 37 patients were in remission phase, while the other 14 patients presented a disease exacerbation. The acute phase of the autoimmune diseases was confirmed also on the basis of the evidence of abnormally high CRP levels. The clinical characteristics of patients are reported in Table 1. For the immune evaluation, venous blood samples were drawn in the morning after an overnight fast. Normal values obtained in our laboratory (95% confidence limits) were above 1.500/ mm3 for lymphocytes, below 450/mm3 for monocytes, and more than 2.1 for LMR. The results were compared to those observed in a control group of 100 sex- and age-matched healthy subjects. Data were reported as mean +/- SE, and statistically analyzed by the Student’s t test, and the chi-square test, as appropriate.
Results
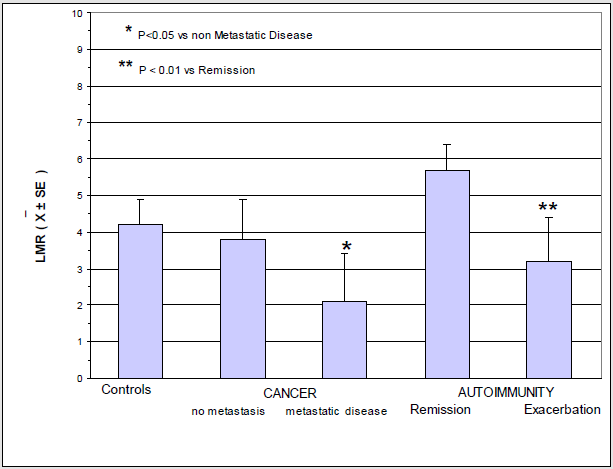
Lymphocytopenia occurred in 23/81 (28%) metastatic cancer patients, and in only 4/68 (6%) patients with locally limited disease. This difference was statistically significant (P<0.01). On the contrary, monocytosis was observed in 15/81 (19%) metastatic patients, and in only 2/68 (3%) non-metastatic patients. Also this difference was statistically significant. On the other side, lymphocytopenia occurred in only 2/37 (5%) patients with autoimmune disease in remission phase, and in 9/14 (64%) patients with active disease. This difference was statistically significant (P<0.05). Finally, the percentage of monocytosis observed in patients with active autoimmune disease was statistically significant greater than that found in patients in remission phase of disease (3/37 (8%) vs 11/14 (79%), P<0.01). Mean values of LMR observed in cancer and autoimmunity in relation to the different phases of disease are illustrated in Figure 1. As shown, LMR mean values observed in metastatic cancer patients were significantly lower with respect to those observed in both controls (P<0.01) and non-metastatic patients (P<0.05). LMR mean values found in patients with autoimmune disease in remission phase were higher than those observed in controls, without, however, statistically significant differences, while they were significantly higher than those found in patients with active autoimmune pathology (P< 0.01).
Discussion
According to previous data reported in the literature [1-4], this
study would suggest that lymphocytopenia and monocytosis may
occur in both metastatic cancer patients, and in patients with active
autoimmune disease, even though the too low number of patients
does not allow us to identify possible different LMR profiles in
relation to the different tumor histotypes and to the various
autoimmune pathologies. This apparently paradoxical immune
behavior could mainly depend on TGF-beta blood concentrations,
as well as on its interactions with IL-17, IL-6, IL-2, and IL-12
secretions. In more detail, TGF-beta blood levels have appeared to
be abnormally high in advanced neoplasms [10] and abnormally
low in autoimmune diseases [11]. Moreover, it has been shown
that the increase in TGF-levels may predict a worse prognosis in
metastatic cancer patients [10], whereas it has appeared to express
a control of disease in the autoimmune pathologies [11]. TGFbeta
alone would inhibit IL-17 secretion, whereas TGF-beta in
association with IL-6 may promote TH17 cell differentiation, with
a consequent enhanced IL-17 production [12]. Then, IL-6 levels,
which are increased in inflammatory conditions, would constitute
the main factor responsible to pilot the effect of TGF-beta on IL-
17 secretion in an inhibitory, or in a stimulatory way. On the
other side, IL-6 and IL-17 are connected by reciprocal stimulatory
actions, then by a complex positive feedback mechanism [13].
Another controversial molecule is IL-2 itself, which is released
from TH1 lymphocytes [14], since IL-2 may either predispose
to the autoimmunity by widely activating the whole immune
system in a non-specific manner, or counteract the development of
autoimmune processes by stimulating T reg cell functions, with a
following enhanced production of TGF-beta [15], and inhibiting IL-
17 secretion [16].
Finally, IL-12 is also essential for the modulation of the immune
functions [17], namely in the autoimmune pathologies, since it
may inhibit the secretion of both TGF-beta [18] and IL-17 [19]
secretions. The inhibition of TGF-beta secretion may predispose
to the autoimmune processes, whereas the inhibition of IL-17
secretion would counteract the onset of autoimmune diseases.
In addition, IL-12 stimulates TH1 cell differentiation and IL-2
production [17], which in turn may exert opposite effects on the
autoimmune mechanisms [14-16]. Therefore, only a concomitant
valuation of at least TGF-beta, IL-6, IL-17, IL-2, and IL-12 blood
concentrations in relation to LMR could clarify the immune
mechanisms and the functional status of the cytokine network in
the autoimmune pathologies. In any case, it must be constantly
remarked that IL-2 represents the most important human growth
factor for lymphocytes, namely for T cells [14], then lymphocyte
count would namely depend on IL-2 blood concentrations.
Therefore, the evidence of both increase and decrease in IL-2 blood
levels in the autoimmune diseases is already sufficient to explain
the occurrence of both lymphocytosis and lymphocytopenia in the
autoimmune diseases reported in the literature, depending on the
different phases of disease. On the contrary, the immune behavior
occurring in the neoplastic disease is more clear, since it has been
shown that the metastatic disseminated tumors are constantly
characterized by a progressive decline in IL-2 blood concentrations, successively followed by a concomitant decline in IL-12 secretion
[20], in association with an increase in TGF-beta and IL-6 levels,
which are responsible for cancer-related immune suppression,
while IL-17 profile in cancer needs to be better investigated and
defined. In any case, the results of this preliminary study require to
be confirmed and more explained by monitoring changes in LMR
values in the same patients in relation to the clinical course of their
disease.
References
- Riesco A (1970) Five-yearcancer cure: relation to totalamount of peripherallymphocytes and neutrophils. Cancer 25: 135-140.
- Gu L, Li H, Chen L, Ma X, Li X, et al. (2016)Prognosticrole of lymphocyte-to-monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget 3: 7876-7881.
- Lissoni P, Messina G, Rovelli F, VigoréL, Lissoni A, et al. (2018) Low lymphocyte-to-monocyte ratio isassociated with an enhancedregulatory T lymphocytefunction in metastaticcancerpatients. Int J RecAdv Multi Res 5: 3353-23356.
- Du J, Chen S, Shi J, Zhu X, Ying H, et al. (2017) The associationbetween the lymphocyte-to-monocyte ratio and disease activity in rheumatoidarthritis. ClinRheumatol 36(12): 2689-2695.
- Brivio F, Fumagalli L, Parolini D, Messina G, Rovelli F, et al. (2008) T helper/ T regulatorlymphocyte ratio as a new immunobiological index to quantify the anticancer immune status in cancerpatients. In Vivo 22(5): 647-650.
- Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-relatedinflammation. Nature 454(7203): 436-444.
- Grivennikov SI, Greten FR, Karim M (2010) Immunity, inflammation, and cancer. Cell 140(6): 883-899.
- Sakaguchi S, Wing K, Onishi Y, Prieto Martin P, Yamaguchi T (2009) Regulatory T cells: how do theysuppress immune responses?Int Immunol 21(10): 1105-1111.
- Lissoni P, Messina G, Cenaj W, Rovelli F, Porro G, et al. (2018) The role of IL-17 secretion in mediating the influence of stress on cancer and othersystemicdiseases. MOJ LympholPhlebol 2(1): 31-34.
- Connolly EC, Freimuth J, Akhurst RJ (2012) Complexities of TGF-beta targetedcancer therapy. Int J Biol Sci 8(7): 964-978.
- Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK (2006) Role of IL-17 and regulatory T lymphocytesinasystemicautoimmnedisease. J ExpMed 203(13): 2785-2791.
- McKenzie BS, Kastelein RA, Cua DJ (2006) Understanding the IL-23 – IL-17 immune pathway. TrendsImmunol 27(1): 17-23.
- Kryczek I, Wei S, Vatan L, EscaraWilke J, Szeliga W, et al. (2007) Cutting edge: opposite effects of IL-1 and Il-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol 179(3): 1423-1426.
- Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA (1982) Lymphokine-activated killer cellphenomenon. J ExpMed 155(6): 1823-1841.
- Humrich J, Riemekasten G (2019) Low-dose interleukin-2 therapy for the treatment of systemic lupus erythematosus. CurrOpinRheumatol 31(2): 208-212.
- Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, et al. (2007) Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26(3): 371-381.
- Banks RE, Patel PM, Selby PJ (1995) Interleukin-12: a new clinical player in cytokine therapy. Br J Cancer 71(4): 655-659.
- King IL, Segal BM (2005) Cutting edge: IL-12 induces CD4+CD25- T cellactivation in the presence of T regulatorycells. J Immunol 175(2): 641-645.
- Hoeve MA, Savage ND, De Boer T, Langenberg DM, De WaalMalefyt R, et al. (2006) Divergenteffects of IL-12 and IL-23 on the production of IL-17 by human T cells. Eur J Immunol 36(3): 661-670.
- Lissoni P, Messina G, Tantarelli R, Lissoni A, Tantarelli O, et al. (2017) The psychoneuroimmunotherapy of human immune-mediatedsystemicdiseases, includingcancer and autoimmune diseases. J MolOncol Res 1:7-13.

 Research Article
Research Article