Abstract
Background: Metformin is used as a first-line treatment in patients with type 2 diabetes mellitus and have a significant impact on the prevention of complications associated with type 2 diabetes mellitus.
Aim: To evaluate the effect of metformin treatment on vitamin B12 level and associated events in patientsliving with type 2 diabetes mellitus and further evaluate the efficacy of this drug in the prevention ofcardiovascular disease associated with type 2 diabetes mellitus.
Methods: A literature search was conducted on PubMed-Medline database up to the 07 March 2020. Elevenstudies investigating the impact of metformin in type 2 diabetes mellitus were included in final synthesis after critical evaluation.
Result: Metformin increases risk of vitamin B12 deficiency OR, 95%CI[2.41 [1.58,3.68], p<0.0001],heterogeneity[Chi2=2.47, I2=0%, p=048]; the risk of stroke, retinopathy, anaemia, neuropathy andmyocardial infarction [1.05[0.94,1.18]], heterogeneity [Chi2=3.58, I2=0%, p=0.76].Additionally, metformin treatment was not associated with CVD including hypertension in T2DM OR,95%CI [0.92[0.73,1.16], heterogeneity [Chi2=93.18, I2=89%, P<0.00001].
Conclusion: In this meta-analysis, we conclude that metformin in patients with type 2 diabetes mellitus is associatedwith an increased risk of vitamin B12, stroke, retinopathy, anaemia, neuropathy and myocardialinfarction. Secondly, it prevents cardiovascular diseases and hypertension.
Keywords: Metformin; Type 2 Diabetes Mellitus; Vitamin B12, Cardiovascular Disease, Neuropathy
Introduction
Type 2 diabetes mellitus [T2DM] is a metabolic condition
characterised by hyperglycaemia resultingfrom insulin resistant
or impaired insulin function [1]. T2DM have a high risk of
developingcardiovascular disease [CVD] compared to individuals
without diabetes [2,3]. The pharmacological therapies currently
used in the prevention of secondary complications associatedwith
T2DM include metformin; this class of drugs improve insulin
sensitivity and reduce body weight [4]. Although this drug provides
therapeutic benefits in T2DM patients and associated CVD, there
areother concerns it poses to individuals using it, including
decreasing serum vitamin B12 levels [5]. In aclinical setting,
when it is not detected early or if there is an incorrect diagnosis,
this deficiency remainsuntreated, resulting in severe deficiency.
Ultimately, this causes megaloblastic anaemia, alteration ofmental
states and neurological damage [5-7]. In most cases, diabetic
neuropathy symptoms can overlap with pricking, impaired
vibration and musclesensation [8].
Thus, peripheral neuropathy as a result of vitamin B12
deficiency may contribute to theaggravation of diabetic peripheral
neuropathy [6,7]. The progression of neurologic damage due
tovitamin B12 deficiency can be treated if diagnosed early
with the administration of vitamin B12 [9]. However, if there is misdiagnosis, permanent neurological damage may not be reversed
[7]. Additionally, these patients present with gastric abnormalities,
including diarrhoea, nausea, and loss ofappetite, others may
present with a sore and reddened tongue. These abnormalities
may result in anunexpected reduction in body weight. Similarly,
hepatomegaly and jaundice around the eyes and skinmay also be
observed [10]. Although previous studies have reported vitamin
B12 deficiency associated with metformin, there iscontradicting
outcomes presented. Moreover, some studies show no association
[11]; others areshowing positive association. Therefore, we aimed
to conduct a first meta-analysis on the effect ofmetformin treatment
on vitamin B12 level and associated events in patients living with
type 2 diabetesmellitus. Furthermore, to assess the efficacy of this
drug in ameliorating cardiovascular diseaseassociated with type 2
diabetes mellitus.
Material and Methods
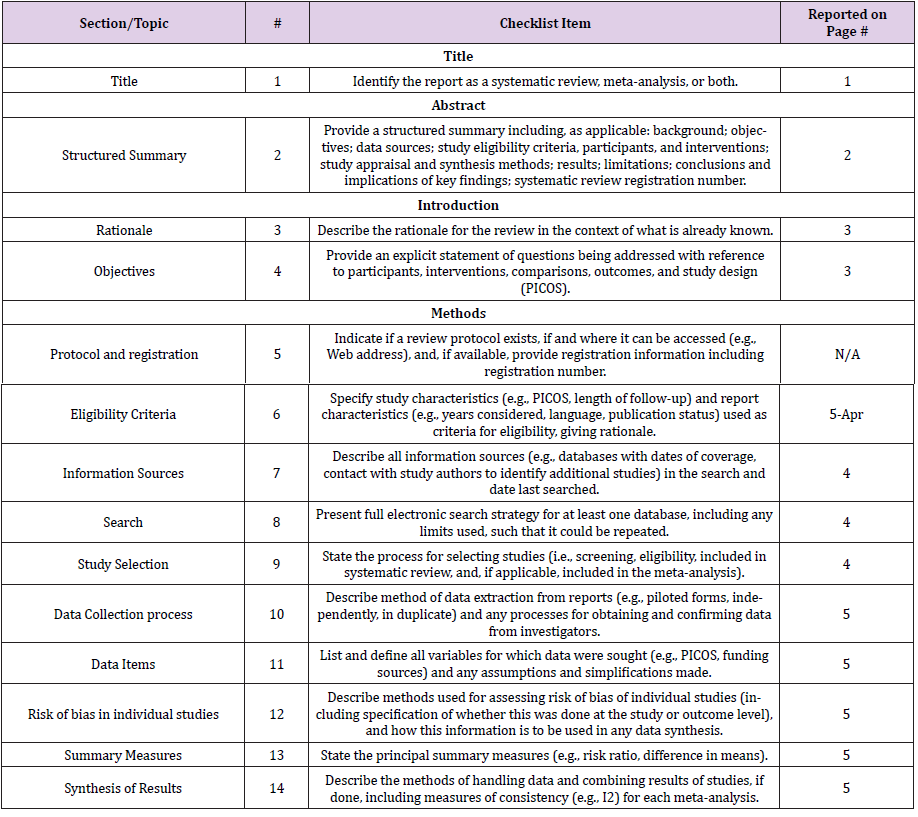
Preferred Reporting Item for Systematic Reviews and Meta-Analysis [PRISMA] Guidelines [12]
was used when preparing this meta-analysis [Appendix file 1]. The ethics approval was not needed as this study only assess data extracted from already published studies.
Research question
Does metformin treatment alleviate type 2 diabetes mellitus related adverse events?
Search Strategy and Information Source
PubMed-Medline electronic database was used to search for published literature using the MedicalSubject Headings [MeSH] terms ‘‘metformin’’, “vitamin B12 deficiency” and ‘‘diabetes mellitus’’without language restriction. The studies meeting eligibility criteria were subjected to critical evaluationand included in the final synthesis. The search was for studies published since inception until 07 March2020. The exact search strategy is attached in appendix 1.
Study Selection
The selection procedure was conducted by two authors
independently [KM and MSM]. Referencemanager software,
Mendeley Desktop version 1.19.4 [Elsevier, Amsterdam,
Netherlands] was used tostore retrieved studies. Firstly, we
screened studies based on the title and abstract for relevance.
Subsequently, the full-text studies were retrieved and critically
evaluated for eligibility in the systematicreview and meta-analysis.
Additionally, we screened the bibliographical lists of included
studies toidentify additional eligible studies that might have been
missed on electronic database search.
PECO: P: patients with type 2 diabetes mellitus; E: diabetes
status; C: healthy control participants; O: adverse events, includingcardiovascular disease, neuropathy, nephropathy, myocardial
infarction,hypertension, stroke and anaemia.
Eligibility Criteria
Inclusion Criteria: Randomised control trials, cross-sectional, prospective or retrospective observational and cohortstudies. Mainly studies reporting on the impact of metformin treatment on the development of adverseevents including vitamin B12 deficiency, neuropathy, nephropathy, hypertension, anaemia, myocardialinfarction, retinopathy and stroke were included. Study selection was carried out by KM and MSM, andwhere there was disagreement, the same authors reached a conclusion through discussion and reevaluatingthe study.
Exclusion Criteria: We excluded editorials, letters to editors, case reports, reviews, the study on other treatment other than metformin and studies with no proper control.
Data Extraction and Data Items
Two independent authors [KM and MSM] reviewed each abstract, retrieved full-text studies and extracted information from each study. Relevant data items extracted from each study were primaryauthor surname and year of publication, the country, study design, population size, age and events. Where there was disagreement between the two independent authors, a resolution was reached through discussion and consensus and re-evaluation of study in question. Mendeley reference manager version (1.19.4) software [Elsevier, Amsterdam, Netherlands] was used to save collected data.
Assessment of Risk and Quality
The assessment of the quality of each included study was evaluated using a standard score by independent authors investigators. Cochrane tool was used to assess the risk of bias and quality of the studies. The disagreement between the two independent authors was resolved through discussion and reevaluation of study in question.
Data Synthesis and Analysis
The Review Manager [RevMan] version 5.3 software [The Nordic Cochrane Centre, The Cochrane Collaboration, 2014] data analysis was used to carry out all the dichotomous data analysis. To determine the odds ratio [OR], the number of events and the total number of participants in the metformin and placebo group were computed. Effects measures were reported as OR and 95% confidence intervals [CI]. OR<1, OR=1, OR>1, classified as not associated with exposure of metformin, metformin not affecting odds of adverse events and metformin-associated with higher odds of adverse events respectively [13]. Heterogeneity was tested with Cochrane chi-square statistics and measured with the Higgins [I2] statistic tests [14]. In the case where heterogeneity was observed, an attempt to find sources of heterogeneity was made through subgroup analysis and sensitivity tests. The I2 = 0%, I2 = 50%, were no and substantial level of heterogeneity respectively. Considering high clinical heterogeneity, a random effects model was used for meta-analyses in case of substantial heterogeneity while the fixedeffect model was used for studies which showed no presence of heterogeneity. Subgroup analyses were performed according to different events, including CVD, anaemia, neuropathy, retinopathy, nephropathy, stroke, hypertension and vitamin B12 deficiency. Publication bias was visually assessed using the funnel plots [symmetrical shape demonstrating an absence of publication bias]. A probability values of less than 0.05 were considered significant statistically.
Result
Selected Studies
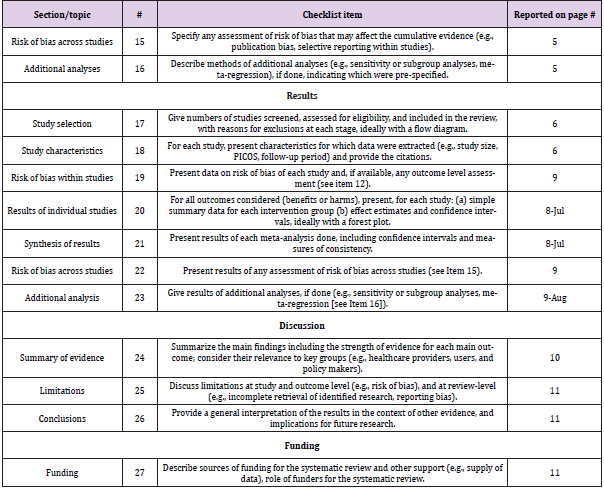
A search on PubMed database yielded 64 studies, based on our eligibility criteria 12 studies [5,15,16-23,24] were critically analysed and further included in the final synthesis (Figure 1).
Overview of the Included Studies
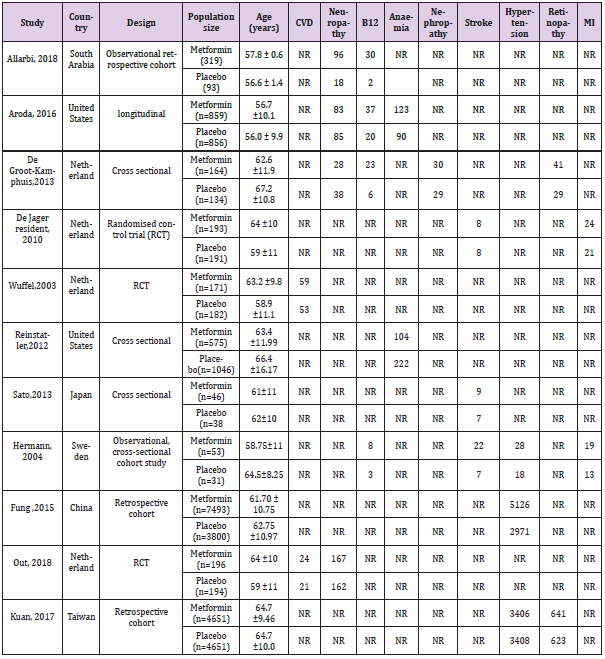
All included studies were published in peer-review journals from 2003 to 2018, and their characteristicsare shown in Table 1. The included studies comprised of 25936 participants, 14720 [56.8%] of whomwere T2DM on metformin, and 11216 [43.2%] were T2DM on placebo. The sample size of includedstudies ranged between 31 and 7493 participants. Among the included 11 studies, three wererandomized control trials; three were cross-sectional, two retrospective cohorts, one observationalretrospective cohort, one longitudinal study and one study that was a mixture of an observational, crosssectionalcohort. These studies were conducted all over the world with four studies conducted inNetherland, two in the United States, one in each of the following country [China, Japan, south Arabia,Sweden, and Taiwan] (Table 1S -3S).
Table 1S: Characteristics of included studies.
Note: CDV: Cardiovascular Disease, B12: Vitamin B12, MI: Myocardial Infarction.
Data Synthesis
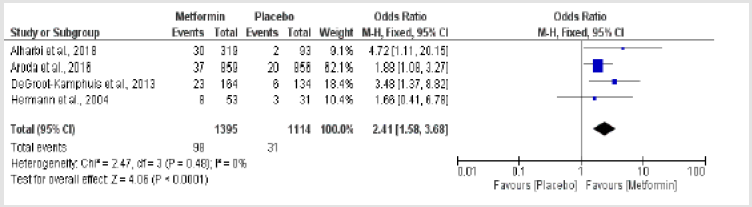
Vitamin B12 In Type 2 Diabetes Mellitus Patients on Metformin Treatment: The current meta-analysis has shown that T2DM patient on metformin has an increased risk ofdeveloping vitamin B12 deficiency when compared to healthy patient OR, 95%CI[2.41 [1.58,3.68],p<0.0001], interestingly the analysed studies showed no level of heterogeneity[Chi2=2.47, I2=0%,p=048] (Figure 2).
Figure 2: Metformin-induced vitamin B12 deficiency in type 2 diabetes mellitus versus patients onplacebo.
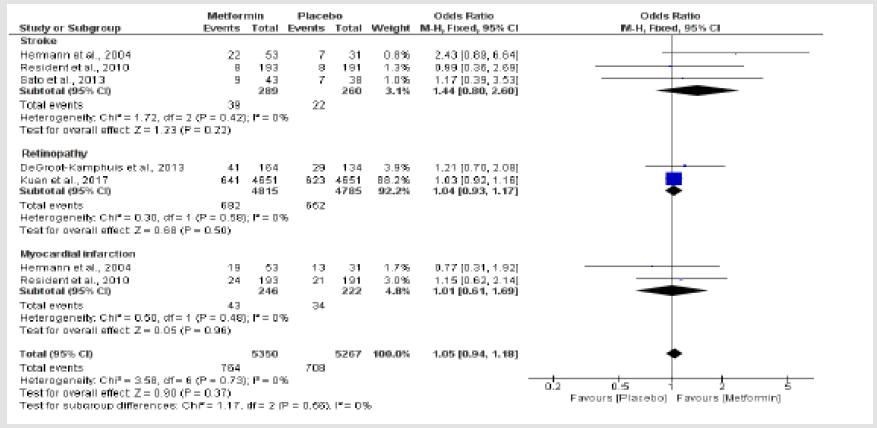
Adverse Events Induced by Metformin Treatment in Type 2 Diabetes Mellitus: According to the current study, patient with type 2 diabetes mellitus on metformin have increased riskof been attacked by stroke compared to T2DM not on metformin treatment OR, 95%CI[1.44 [0.80,2.60], Chi2=1.72, I2=0%, p=0.42], retinopathy [1.04[0.93, 1.17], Chi2=0.30,I2=0%, p=0.58], myocardialinfarction [1.01[0.61,1.69], Chi2=0.50, I2=0%, p=0.96]. The overall effect estimates for these adverseevents also indicated that metformin is associated with an increased risk of stroke, retinopathy andmyocardial infarction [1.05[0.94,1.18]] of interest was absence of heterogeneity which was alsoconfirmed by subgroup analysis[Chi2=1.17, I2=0%, p=0.56] (Figure 3).
Figure 3: Metformin-induced adverse events in type 2 diabetes mellitus compared to diabetes patientson placebo.
Other Adverse Events Induced by Metformin Treatment: Current metanalysis shows that metformin treatment in type 2 diabetes mellitus patients reduces therisk of developing cardiovascular disease OR, 95%CI [0.81[0.31,2.10], Chi2=5.86, I2=83%, p=0.02],including hypertension [0.78[0.50,1.22], Chi2=57.79, I2=97%. P<0.00001] however shows an increasedrisk of neuropathy [1.00[0.65,1.55], Chi2=9.68, I2=69%] and anaemia [1.08[0.63,1.85], Chi2=7.72,I2=87%, p=0.0005] (Figure 4). The overall pooled effect estimate revealed that metformin treatment isnot associated with these adverse events in T2DM [0.92[0.73,1.16], Chi2=93.18, I2=89%, p<0.00001],as a result of moderate level of heterogeneity, subgroup analysis was performed and it revealed noheterogeneity [Chi2=1.05, I2=0%, p<0.00001] (Figure 4).
Figure 4: cardiovascular disease and related adverse events triggered by metformin treatment in type2 diabetes mellitus compared to placebo.
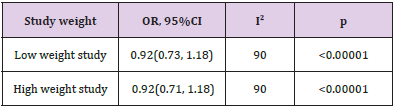
Sensitivity Analysis for Studies That Showed A Substantial Level Of Heterogeneity: A sensitivity test was performed by removing one study at a time, and recalculating the effect measures,by removing low weight study, the OR did not change; however, the direction of confidence intervalslightly changed from [0.92[0.73, 1.16] to [0.92[0.73,1.8]], similarly when high weight study wasremoved we noted a slight change in confidence interval [0.92[0.71,1.18]] (Table 1).
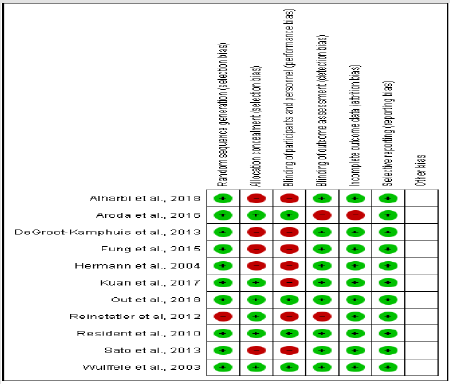
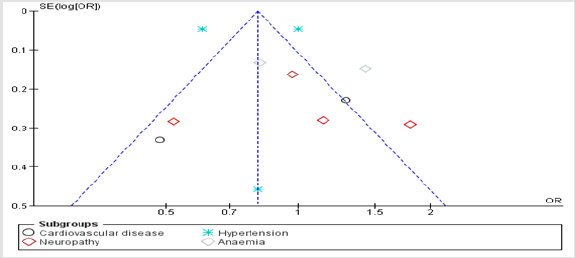
Risk of Bias and Quality Assessment: Studies were scored as good quality when it has four or more positive, which showed a low risk of biasor fair if it has three scores out of six domains. Three studies scored all six positive, thus low risk ofbias in all domains. Four scored four out of possible six domains with two domains classified as a highrisk this included allocation and blinding of participants and personnel. One study was rated high riskin terms of the blinding and incomplete outcome however other four domains were of low risk, andlastly, one study had a fair quality as it has scored 3 points out of possible six domains (Figure 5 & Figure 1S-4S).
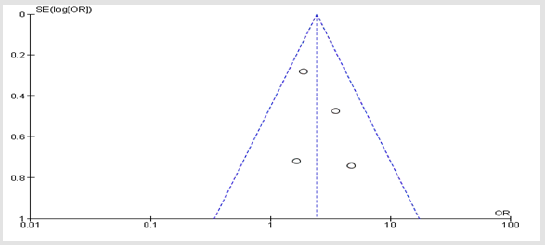
Figure 1S: Symmetrical presentation using funnel plot of vitamin B12 amongst the included studies showing no publication bias.
Publication Bias
The risk of publication bias was assessed graphically by use of funnel plots, and amongst all adverseevents in the included studies, there was no evidence of publication bias trough graphical presentationsof funnel plots as indicated in (Figures 1S-3S).
Discussion
This is the primary meta-analysis to evaluate the effect of metformin on the development of adverseevents in patients with type 2 diabetes mellitus. With the current high prevalence of T2DM andcardiovascular disease worldwide we found it necessary to synthesise the current meta-analysis onmetformin treatment and risk of adverse events, to highlight and weigh the therapeutic benefits ofmetformin and its adverse effects in patients with type 2 diabetes.Metformin has been prescribed worldwide as first-line treatment in patients with type 2 diabetesmellitus [16,25], and have a significant impact in reducing secondary complications associated with T2DM. The findings obtained from this metaanalysis included a high risk of developing vitamin B12deficiency associated with metformin treatment. Vitamin B12 deficiency if is prolonged lead tosubsequent adverse events, including neuropathy and anaemia [15-17,20,22,23], and this was evidentin the current study as revealed increased risk of neuropathy and anaemia associated with metformintreatment.This further supports previously published studies which demonstrated an increased risk of anaemiaand neuropathy arising from metformin-induced vitamin B12 deficiency [5,16,17]. Diabetic neuropathycan also manifest other symptoms, including impaired vibration and muscle sensation [8]. Thus,peripheral neuropathy induced by vitamin B12 deficiency due to metformin treatment may contribute tothe severe diabetic peripheral neuropathy [6,7]. The progression of neurological trauma associated withvitamin B12 deficiency is treatable if diagnosed early with the administration of vitamin B12 [9].However, if there is misdiagnosis, neurological damage may not be reversed [7].
Diabetic patients frequently present with anaemia which is common blood disorders resulting intodevelopment and exacerbation into micro and macrovascular complications [26]. It is considered anindicator of kidney disease and increases the risk of developing cardiovascular disease [CVD][27,28]As the first line of defence against cardiovascular disease, it was also revealed in our meta-analysis thatmetformin could alleviate cardiovascular disease, including hypertension. However, we observed anincreased risk of stroke, retinopathy, and myocardial infarction associated with metformin treatment.Our results are suggesting that metformin treatment has therapeutic benefits in terms of preventing CVDin patients living with T2DM; however, we suggest its impact on vitamin B12 levels not to be overlookedas it might result into the manifestation of other complications including neuropathy and anaemia.
Conclusion
Based on the finding synthesised in this meta-analysis, we have shown that type 2 diabetes mellituspatients on metformin treatment have a high risk of developing vitamin B12 deficiency which furtherpredisposes them to neuropathy, and other related adverse events including anaemia, retinopathy, nephropathy, stroke and myocardial infarction, of importance, is its beneficial effects in reducingcardiovascular disease including hypertension.
Strength and Limitation
One of the limitations includes different study design amongst the included studies. With that been said,the study has its strength which ranges from good quality of studies been included. The literature searchwas from inception until 07 march, to make sure we get old studies with background knowledge aboutmetformin and its impacts on type 2 diabetes. Pooling all these studies have increased the statisticalpower as compared to individual study. The combined sample size was sufficient; thus, we can concludethat our meta-analysis was not statistically underpowered. The studies showed no heterogeneity in manyadverse events, and the sensitivity analysis also showed a slight change in effect measures and thefunnels plots showed no presence of publication bias amongst the included studies. The studies werepublished in different regions of the world.
Ethical Considerations
Not required as this is review and analysis of studies that are already published.
Source of Funding
The study was partially funded by the National Research Foundation of South Africa [NRF, grantno:121496].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships thatcould have appeared to influence the work reported in this manuscript.
Author Contribution
KM and MSM contributed equally from conceptualisation, screening, analysis, first draft to the finalapproval for publication of this manuscript.
References
- Kahn CR (1994) Banting lecture: Insulin action, diabetogenes, and the cause of type II diabetes.Diabetes43(8):1066-1084.
- Mokgalaboni K, Dludla PV, Nyambuya TM, Yakobi SH, Mxinwa V, et al. (2020) Monocyte-mediated inflammation and cardiovascular risk factors in type 2 diabetes mellitus: A systematic review and meta-analysis of pre-clinical and clinical studies.
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, et al. (1999) The online versionof this article, along with updated information and services, is located on the World Wide Web:1134-1146.
- Domecq JP, Prutsky G, Leppin A, Sonbol MB, Altayar O, et al. (2015) Drugs commonlyassociated with weight change: A systematic review and meta-analysis. J Clin EndocrinolMetab100(2):363-370.
- De Jager J, Kooy A, Lehert P, Wulffelé MG, Van Der Kolk J, et al. (2010) Long termtreatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency:Randomised placebo controlled trial. BMJ340(7757):1177.
- Bell DSH (2010) Metformin-induced vitamin B12 deficiency presenting as a peripheral neuropathy.South Med J103(3):265-267.
- Pierce SA, Chung AH, Black KK (2012) El seguimiento a las concentraciones de vitamina B12 enuna población de veteranos que utilizandosisaltas de metformin por periodos largos detiempo. Ann Pharmacother46(11):1470-1475.
- Pflipsen MC, Oh RC, Saguil A, Seehusen DA, Topolski R (2009) The prevalence of vitamin B12deficiency in patients with type 2 diabetes: A cross-sectional study. J Am Board Fam Med22(5):528-534.
- Lindenbaum J, Healton EB, Savage DG, Brust JC, Garrett TJ, et al. (1988) Stabler SP AR. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemiaor macrocytosis. N Engl J Med318:1720-1728.
- (2008) Disorders, NORD: National Organization for Rare Disoders. Anemia, Megaloblastic p. 1-7.
- Elhadd T, Ponirakis G, Dabbous Z, Siddique M, Chinnaiyan S, et al. (2018) Metformin use isnot associated with B12 deficiency or neuropathy in patients with type 2 diabetes Mellitus inQatar. Front Endocrinol (Lausanne) 9:2-6.
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviewsand meta-analyses: the PRISMA statement. J Clin Epidemiol62(10):1006-1012.
- Sedgwick P, Marston L (2010) Statistical question: Odds ratios. BMJ341(7769):407.
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in metaanalyses.BMJ 327(7414):557-560.
- Alharbi TJ, Tourkmani AM, Abdelhay O, Alkhashan HI, Al-Asmari AK, et al. (2018) The association of metformin use with vitamin B12 deficiency and peripheral neuropathy inSaudi individuals with type 2 diabetes mellitus. PLoS One13(10):e0204420.
- Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, MarcovinaSM, et al. (2016) Long-term metformin use and vitamin B12 deficiency in the diabetes prevention programoutcomes study. J Clin Endocrinol Metab101(4):1754-1761.
- DM De Groot-Kamphuis , PR Van Dijk, KH Groenier, ST Houweling, HJG Bilo, et al. (2013) Vitamin B12 Deficiency Among Type 2 Diabetes Patients UsingMetformin. Neth J Med71(7):386-390.
- Fung CSC, Wan EYF, Wong CKH, Jiao F, Chan AKC (2015) Effect of metformin monotherapy oncardiovascular diseases and mortality: A retrospective cohort study on Chinese type 2 diabetesmellitus patients. Cardiovasc Diabetol14(1):1-14.
- Hermann LS, Nilsson B, Wettre S (2004) Vitamin B12 status of patients treated with metformin: Across-sectional cohort study. Br J Diabetes Vasc Dis4(6):401-406.
- Kuan YC, Huang KW, Lin CL, Hu CJ, Kao CH (2017) Effects of metformin exposure onneurodegenerative diseases in elderly patients with type 2 diabetes mellitus. Prog Neuro-Psychopharmacology Biol Psychiatry 79(2):77-83.
- Metaxas C, Zurwerra C, Rudofsky G, Hersberger KE, Walter PN (2018) Impact of type 2 Diabetesand Metformin use on Vitamin B12 Associated Biomarkers - An Observational Study. ExpClin Endocrinol Diabetes126(6):394-400.
- Out M, Kooy A, Lehert P, Schalkwijk CA, Stehouwer CDA (2018) Long-term treatment withmetformin in type 2 diabetes and methylmalonic acid: Post hoc analysis of a randomizedcontrolled 4.3-year trial. J Diabetes Complications 32(2):171-178.
- Reinstatler L, Qi YP, Williamson RS, Garn JV, Oakley GP (2012) Association of biochemical B 12deficiency with metformin therapy and vitamin B 12 supplements: The National Health andNutrition Examination Survey, 1999-2006. Diabetes Care35(2):327-333.
- Sato Y, Ouchi K, Funase Y, Yamauchi K, Aizawa T (2013) Relationship between metformin use,vitamin B12 deficiency, hyperhomocysteinemia and vascular complications in patients withtype 2 diabetes. Endocr J60(12):1275-1280.
- Calvo Romero JM, Ramiro Lozano JM (2012) Vitamin B12 in type 2 diabetic patients treated withmetformin. Endocrinol y NutrEnglish Ed 59(8):487-490.
- Thomas MC, Macisaac RJ, Tsalamandris C, Molyneaux L, GoubinaI, et al.(2004) Theburden of anaemia in type 2 diabetes and the role of nephropathy:A cross-sectional audit.19(7):1792-1797.
- Dikow R, Schwenger V, Scho M, Ritz E (2002) How should we manage anaemia in patients withdiabetes?67–72.
- Bosman, Winkler AS, Marsden JT, MacdougallIc, Peter J Watkins (2001) Anemia with Erythropoietin Deficiency Occurs Early in Diabetic Nephropathy DEBORAH24(3).

 Research Article
Research Article