Abstract
Polylactic-co-glycolic acid (PLGA) microparticles have attracted scientists and engineers from various research fields. PLGA microparticles have been extensively used for drug delivery since the synthesized polymer is FDA approved polymer. Here, we present a method to control the formation of PLGA microparticles. prose and the surface activation of microparticles. A series of nanoporous PLGA microparticles were synthesized with different surface porosity. The porosity agent concentration influenced the surface activation. Thus, nanoporous PLGA microparticle can be introduced as a new focused drug delivery platform. The porosity of the activated microparticles showed a direct relationship with the covalent attachment of Lysine primary amine molecule. The nanoporous PLGA microparticle synthesis is a fast and low-cost method. Furthermore, nanoporous PLGA microparticles are suitable for applications in targeting one or multiple extracellular ligands. In specific, cancer cell ion channels exist in the cell ‘s extracellular space and can be targeted without entering the target cells.
Keywords: Cell Targeting; PLGA; Ion Channels; Nanoporous; Target Delivery; Salt-Leaching.
Introduction
PLGA has been widely used for drug delivery and shown
significant improvement in therapeutic areas. PLGA blocks are
built from lactic and glycolic acid monomers that can be absorbed
by the cell. Therefore, PLGA is a non-immunogenic, non-toxic, and
stable polymer for the development of drug delivery. Furthermore,
hydrophilic and hydrophobic drug molecules, DNA, and proteins
can be encapsulated [1]. PLGA has an excellent biocompatibility
profile [2,3]; therefore, the encapsulated molecules achieved target
release profile through degradation and erosion of the polymer
matrix [4,5].
Nanotechnology offers unique and fascinating approaches
in the area of nanomedicine and healthcare application [6,7].
Nanoporous microparticles have been used for bioseparation,
drug release, and tissue engineering. In bioseparation, highly
nanoporous microspheres have been extensively commercialized in
chromatography [8-10]. In drug release, nanoporous microspheres
can be designed to be multifunctional carriers for efficiently loading
drugs [11,12]. The porous nanoparticles showed higher drug
loading and releasing over a period of 30 days from nonporous
nanoparticles [13]. In porous PLGA microparticles, autocatalytic
effects play an essential role in controlling the drug release. The
presence of the nanopores increases the drug molecules’ mobility
and altered the drug release mechanisms [14]. In tissue engineering
and regenerative medicine, nanoporous microspheres are one of
the best candidates for regenerative repair, and tissue engineering
[15,16]. These nanoporous microparticles show significant
advantages in many practical applications.
The primary aims of this study:
(i) Surface activate nanoporous PLGA microparticles (Figure 1),
specifically, the crosslinking of bis (sulfosuccinimidyl) suberate
(BS3) with primary amines to form amide bonds.
(ii) Characterize the effect of salt concentration on the formation
of nanoporous and microparticles porosity.
(iii) study the effects of the porosity on the covalent amide bond
formed between primary amine ligand and BS3 molecules.
We introduce a method that can be used to target extracellular ligand, such as cell ion channels. The channel blockers may crosslink to nanoporous PLGA microparticles and act like a cork in a bottle, preventing the flow of ions in targeted cells until it diffuses off gradually. A targeted cell approach blocking ion channels activity can provide controlled, and a better-tolerated treatment for patients.
Materials and Methods
Materials
All analytical grade reagents used in this work are purchased from Sigma-Aldrich (St. Louis, MO) unless noted otherwise. Poly (D, L-lactide-co-glycolide) 75:25 molar ratio with a carbocyclic group (uncapped) was obtained from Resomer® (Darmstadt, Germany). 1, 2-Dichloroethane (ACS reagent, ≥99%) polyvinylpyrrolidone (PVP-k90), Lysine, and sodium bicarbonate (NaHCO3) were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Micro BCA protein assay kit and bis (sulfosuccinimidyl) suberate (BS3) were purchased from Thermo-Scientific, Rockford, Illinois, USA.
Synthesis of Nanoporous PLGA Microparticles
PLGA microparticles were synthesized using a double emulsion
method [17]. First, 3% (w/v) of the PLGA solution was prepared by
dissolving PLGA in dichloroethane. Then, 0.2 mL of deionized (DI)
water was added to the PLGA solution and vortexed for 30 sec to
create a water-in-oil phase. A solution of 3%, 0.5%, and 0.1% (w/v)
of sodium bicarbonate in DI water were prepared. Then, the sodium
bicarbonate solution was added to the water-in-oil phase sample
to form nanoporous microparticles. Then, 5% of PVP-k90 solution
in DI water was prepared and two mg of BS3 dissolved in DI water
(100 μL) was added to PVP-K90 solution and vortexed for one
minute to ensure well mixing. The step of adding NaHCO3 solution
was skipped for the preparation of nonporous PLGA microparticles,
as shown by the schematic flowchart (Scheme 1). It was then added
dropwise to the stirring beaker of PVP-k90 solution. Table 1 shows
the prepared samples. Next, the solution vials were kept on stir plate
overnight in a chemical hood to allow dichloroethane to evaporate.
Once the dichloroethane evaporated entirely, the solution was
transferred into a 15 ml tube and centrifuged at 4000 rpm for 15
min. The pellets were collected and suspended in phosphate buffer
saline (PBS), then freeze-dried at -80 °C.
The freeze-dried microparticles were taken in a plastic tube,
washed with DI water, and centrifuged at 4,000 rpm for 15 min
three times to form nanoporous PLGA microparticles and remove
the trapped salt. Iterative washing in DI water after centrifugation
is known to remove the water-soluble salts completely [13, 18, 19].
The pellets were suspended in 5 mL PBS. ZetaPALS DLS detector
(Brookhaven Instruments, Holtsville, NY, USA) was used to measure
the size and polydispersity of microparticles
Scheme 1: Schematic flowchart for the procedure to synthesize nanoporous and nonporous microparticles. PLGA microparticles were synthesized using double emulsion method. DI water was added to PLGA solution and vortexed, then NaHCO3 was prepared and added to water-in-oil phase samples. The sample was added dropwise to solution of PVP-K90 with BS3. The sample was kept overnight on stir plate to complete the phase separation. Finally, the sample was washed with DI water to perform salt leaching step.
Preparation of Nanoporous PLGA Microparticles for Scanning Electron Microscope (SEM) Imaging
10 mg freeze-dried microparticles were suspended in 1.5 mL DI water; the sample was diluted for imaging in SEM. DI water was used instead of PBS to avoid crystallization and imaging artifacts stemming from the salts. The sample was sputter-coated with 50 Ǻ thick gold to prevent the surface from getting charged during SEM imaging. The thin layer of gold coating made sure that the glass coverslip surface was conductive enough to image with SEM.
Covalent Attachment of Lysine to Nanoporous PLGA Microparticles
The surface activated nanoporous PLGA microparticles were suspended in PBS for covalent attachment to Lysine. The microparticles underwent the process of conjugation, followed by PBS washing. For each sample in Table 1, 10 mg was suspended in 5 mL PBS, then 30 mg of Lysine was added to each sample and incubated for 30 min. The excess of Lysine was removed by centrifugation followed by PBS wash (x2) to obtain Lysine conjugated to nanoporous microparticles surface.
Results
Nanoporous PLGA Microparticle Morphology
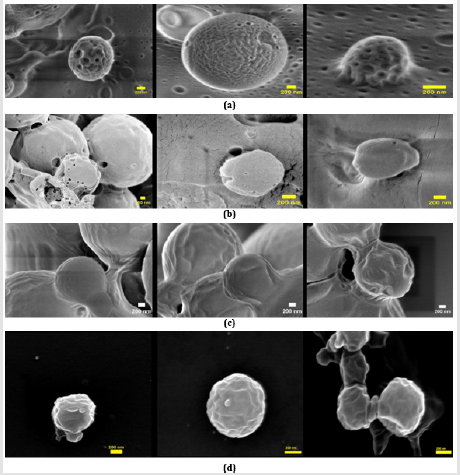
The shape of nanoporous PLGA microparticles is a critical feature in the success of targeting drug delivery [20]. Also, the shape of microparticle influences the surface area available for surface activation. Here, the nanoporous microparticles were prepared using the double emulsion method with NaHCO3 as the pores foaming agent. The morphology of synthesized nanoporous PLGA microparticles was found to be spherical from SEM micrographs (Figure 2). PLGA microparticles with large surface-to-volume ratios are preferred for targeting since sizable surfaces allow for the attaching of many ligands.
Figure 2: SEM images of 3% PLGA microparticles prepared using double emulsion method with different salt concentration. (a) 3% NaHCO3 (b) 0.5% NaHCO3 (c) 0.1% NaHCO3 (d) nonporous PLGA microparticles (Scale bar = 200 nm)
Nanoporous PLGA Microparticle Size
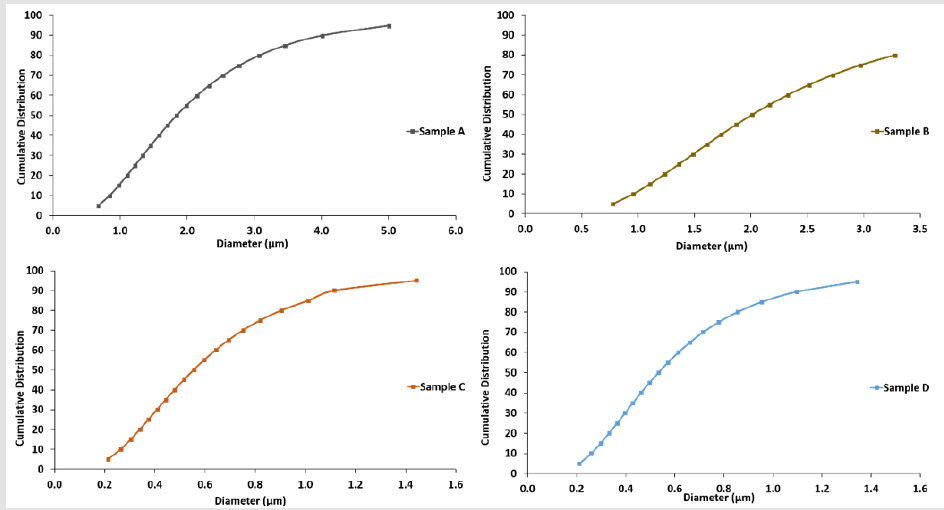
The size distribution of the microparticle samples was measured using a ZetaPALS DLS detector. The mean diameter of the nonporous PLGA microparticles was 0.60 ± 0.30 μm (polydispersity = 0.37). The diameter of nanoporous PLGA microparticles prepared with 0.1%, 0.5% and 3% NaHCO3 was 0.63 ± 0.32 μm (polydispersity = 0.40), 1.80 ± 0.66 μm (polydispersity = 0.40), and 2.11 ± 1.15 μm (polydispersity = 0.45), respectively. The mean diameter of the PLGA microparticles increased with the addition of NaHCO3. Figure 3 shows PLGA microparticle’s cumulative distribution vs. diameter of each sample.
Nanoporous PLGA Microparticle Porosity Analysis
PLGA microparticles samples porosity has been calculated using ImageJ software (National Institutes of Health, Bethesda, Maryland, USA). Using the analyze particles function within ImageJ, the percentage of pixels in the image have been highlighted by applying the built-in image threshold function (Table 2). Since the pores showed a different threshold than the background, porosity can be calculated in a very efficient technique (Figure 4).
Lysine Assay
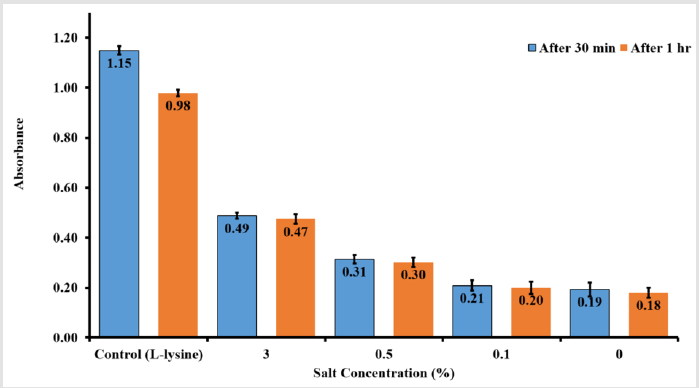
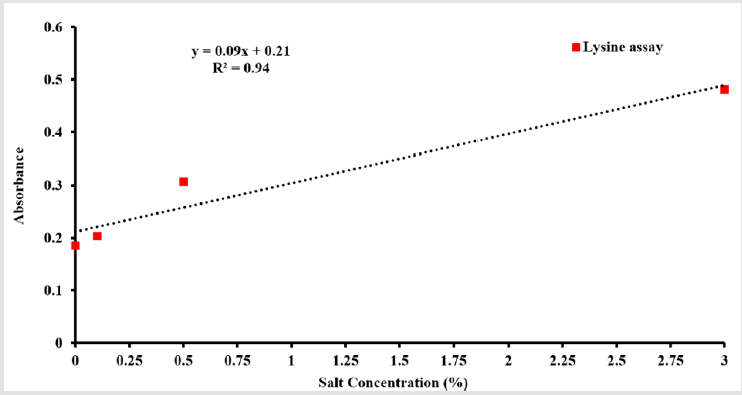
The micro BCA assay kit has been used for colorimetric detection and quantitation of total Lysine molecules have attached to nanoporous PLGA microparticles surfaces. The absorbance of the sample at 562 nm was measured on a plate reader (n =3), with a control sample of Lysine in PBS only (Figure 5). The data has been collected after 30 min and after one hour from Lysine addition to nanoporous PLGA microparticles. The assay results showed a correlation between the amounts of Lysine found in each sample with salt concentration used to prepare the sample. A linear response between the salt concentration and the Lysine binding to nanoporous PLGA microparticles surface with a reported R2 = 0.94 (Figure 6).
Discussion
The spherical morphology of the synthesized nanoporous
PLGA microparticle makes the movement more natural due to
their symmetry; therefore, they travel through indirect pathways
with fewer collisions [21]. No direct measurement has been
conducted to calculate the percentage of PLGA microparticles that
became nanoporous because the PLGA microparticles suspended
in the solution undergo Brownian motion. Therefore, the motion
of the particles is entirely random depends on the size, viscosity,
and temperature. Hence, an estimation of the percentage of PLGA
microparticles became nanoporous by reporting the cumulative
distribution percentage from Figure 3 that reflects mean diameters
from each sample. The result shows ~ 50%, 39.8%, and 50.4% of
Sample A, B, and C, respectively, are nanoporous microparticles.
Controlling the nanoporous PLGA microparticles diameter
size in such an approach can be very useful for the microparticles
applications. For example, platelets (size 2 -3 μm) are known to
promote metastasis by protecting cancer cells against natural killer
cells in the blood, because of direct contact between platelets and
targeted cells [22]. The PLGA microparticles size is very close to
platelets; this can be used to assist the physical attachment to the
targeted cell surface.
Table 2 shows the pore size measurement and porosity
percentage calculation for the samples prepared in Table 1 The
analysis shows an increase in porosity (%) as NaHCO3 concentration
increases. Based on the porosity analysis, the process efficiency
to produce nanoporous depends on the salt concentration. On
the other hand, the pore size is random in this case and does not
depend on the salt’s concentration. One-way ANOVA test shows a
p-value of 0.806, which means no significant difference between
the pore sizes from different samples.
The selective targeting approaches require ligands that
specifically interact with receptors expressed on the cell surface.
Therefore, this means multiple copies of the ligand on the PLGA
microparticles surface to increase the binding effects, which results
in higher affinity. The sample with higher porosity showed higher
Lysine concentration, which can be translated into more primary
amines are attached to the surface. The results show an approach
capable of controlling the density of ligand that could conjugate on
the microparticle surface to enhance the binding, which results in
higher affinity. Also, the results showed a stable covalent attachment
of Lysine to nanoporous PLGA microparticles.
Conclusion
This work demonstrates the method to control the porosity
of nanoporous PLGA microparticles. The nanoporous PLGA
microparticles surface was activated with BS3 to form a stable
amide bond with primary amine groups. The double emulation
technique has been used to synthesize the particles. The BS3
crosslinker chemistry has been used to functionalize the
nanoporous PLGA microparticles surface. This work shows stable
amide bond formation between the BS3 and L-lysine and a novel
method to control the amount of Lysine attachment to the surface
by controlling the porosity of PLGA microparticles. The nanoporous
PLGA microparticles platform can be designed to carry out
multifunctional purposes.
Due to the surface area increase, the functionalization of
microparticles can maximize receptor selectively targeting, which
can be recognized as an advanced therapy for cancer. A higher
density of ion channel blockers or any other ligand would be
available for specific binding and reduce non-specific binding.
The PLGA microparticles have been used to load and deliver drug
molecules. The future direction of this platform is to complete
studies with ion channel blockers to develop the cell-targeted
delivery platform.
Acknowledgment
The authors acknowledge experimental assistance and useful discussion with Dr. M. R. Hassan, Dr. Sai S. Sasank Peri, Dr. Nuzhat Mansur, and Dr. M. U. Raza.
Conflicts of Interest
The authors declare no conflict of interest.
References
- ZhengW (2009) A water-in-oil-in-oil-in-water (W/O/O/W) method for producing drug-releasing, doublewalledmicrospheres. Int J Pharm 374(1-2): 90-95.
- BarrowWW (2004) Microsphere technology for chemotherapy of mycobacterial infections. Curr Pharm Des10(26): 3275-3284.
- KapoorDN, Bhatia A, Kaur R, Sharma R, KaurG, et al.(2015) PLGA: a unique polymer for drugdelivery. TherDeliv 6(1): 41-58.
- ShiveMS, Anderson JM (1997) Biodegradation and biocompatibility of PLA and PLGA microspheres. AdvDrugDeliv Rev 28(1): 5-24.
- FredenbergS Wahlgren M,Reslow M,Axelsson A (2011) The mechanisms of drug release in poly lacticco-glycolic acid)-based drug delivery systems--a review. Int J Pharm 415 (1-2): 34-52.
- CegnarM,Kristl J, Kos J (2005) Nanoscale polymer carriers to deliver chemotherapeutic agents to tumours.Expert Opin Biol Ther 5(12): 1557-1569.
- Juillerat-JeanneretL, Schmitt F (2007) Chemical modification of therapeutic drugs or drug vector systemsto achieve targeted therapy: looking for the grail. Med Res Rev27 (4): 574-590.
- HalaszI, Horvath C (1964) Porous Layer Glass Bead Column Packing in Gas Adsorption LayerChromatography. Anal Chem 36(12): 2226.
- HalaszI, Horvath C (1964) Porous Layer Glass Bead Column Packing in Gas Adsorption LayerChromatography. Anal Chem 36(7): 1178-1186.
- KirklandJJ (1976) Porous Silica Microspheres for High-Performance Size Exclusion Chromatography. JChromatogr 125(1): 231-250.
- LaVanDA, McGuire T, Langer R (2003) Small-scale systems for in vivo drug delivery. Nat Biotechnol21(10): 1184-1191.
- Mohamed F van der WalleCF (2008) Engineering biodegradable polyester particles with specific drugtargeting and drug release properties. J Pharm Sci-Us 97(1): 71-87.
- IlyasA, Islam M, Asghar W, Menon JU,Wadajkar AS,et al. (2013) Salt-LeachingSynthesis of Porous PLGA Nanoparticles. Ieee T Nanotechnol 12 (6): 1082-1088.
- KloseD,Siepmann F,Elkharraz K,Krenzlin S,Siepmann J (2006) How porosity and size affect the drugrelease mechanisms from PLIGA-based microparticles. Int J Pharmaceut 314(2): 198-206.
- HutmacherDW (2000) Scaffolds in tissue engineering bone and cartilage. Biomaterials 21(24):2529-2543.
- ChungHJ, Park TG (2007) Surface engineered and drug releasing prefabricated scaffolds for tissueengineering. Adv Drug Deliver Rev 59(4-5): 249-262.
- AbdallahMG,Yousufuddin M,Yaman S, KhanR, Kim Y, et al. (2017) Surface functionalization ofnanoporous PLGA microparticles. 2017 IEEE 12th Nanotechnology Materials and Devices Conference(NMDC): 204-205
- LeeSB, KimYH, ChongMS, Hong SH, Lee YM (2005) Study of gelatin-containing artificial skin V:fabrication of gelatin scaffolds using a salt-leaching method. Biomaterials 26(14): 1961-1968.
- KimTG, Chung HJ, Park TG (2008) Macroporous and nanofibrous hyaluronic acid/collagen hybridscaffold fabricated by concurrent electrospinning and deposition/leaching of salt particles. ActaBiomater 4(6): 1611-1619.
- KarpF,Busatto C,TurinoL, LunaJ,Estenoz D(2019) PLGA nano- and microparticles for the controlledrelease of florfenicol: Experimental and theoretical study. J Appl Polym Sci 136: (12).
- AlmeriaB, Deng WW, Fahmy TM, Gomez A (2010) Controlling the morphology of electrospraygenerated PLGA microparticles for drug delivery. J Colloid Interf Sci 343 (1): 125-133.
- KocbekP, ObermajerN, CegnarM, Kos J, Kristl J(2007) Targeting cancer cells using PLGA nanoparticlessurface modified with monoclonal antibody. J Control Release 120(1-2): 18-26.

 Research Article
Research Article