Abstract
Background: The efficacy of treatment of Helicobacter pylori (H. pylori) infection has decreased because of increasing resistance to clarithromycin, metronidazole, and levofloxacin. Resistance to amoxicillin is generally low, and high intragastric pH increases the efficacy of amoxicillin, so we performed this study to assess the efficacy of a high-dose dual therapy in treatment-naive patients with H. pylori infection.
Methods: a total of 89 patients with H. pylori infection were recruited in University Medical Center Ho Chi Minh city. All patients underwent endoscopy before treatment. Four to eight weeks after completing the course of therapy, H. pylori infection status was examined by C13 -urea breath tests. Patients were received a high-dose dual therapy (rabeprazole 20 mg QID and amoxicillin 1000 mg QID for 14 days).
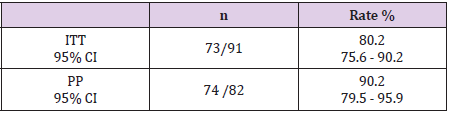
Results: High-dose dual therapy achieved high efficacy of intention-to-treat (ITT) eradication rate, 84.3% (95% CI 75.6 – 91.5%), and of per-protocol (PP) eradication rate 88.8% (95% CI 79.5 – 93.9%). The adverse event rates were 29.2 %. Compliance rate was 96.6%.
Conclusion: A 14day rabeprazole- and amoxicillin-containing high-dose dual therapy achieves a high eradication rate as first-line anti-H. pylori therapy.
Keywords: High Dose Dual Therapy; Eradication; Helicobacter pylori infection
Introduction
The first 3-drug regimen for Helicobacter pylori infection (H.
pylori) including Proton Pump Inhibitor (PPI) / clarithromycin
/ amoxicillin or metronidazole for 7-14 days is not currently
recommended in areas with resistance. clarithromycin> 15%, due
to H. pylori eradication rates drop to unacceptable levels (≤80%)
[1,2]. The main reasons for failure of H. pylori infection include
antibiotic resistance, poor adherence and rapid metabolism of
Proton Pump Inhibitors (PPIs). Clarithromycin resistance is a major
cause of failure of the standard triple regimen. So far, amoxicillinresistant
H. pylori is still rare in the world. Therefore, high-dose
dual therapy amoxicillin-PPI is one of the Maastrich V (2017)
consensus regimens recommended in the treatment of H. H. pylori
infection for the first time [2]. The three most important factors
associated with the success of high-dose dual therapy include the
ability to maintain stomach pH ≥ 6, the dose of amoxicillin and the
interval between doses and duration of treatment. Recently, the
study of Yang et al. Showed that a 14-day high-dose dual therapy
consisting of 20 mg rabeprazole and 750 mg amoxicillin every
6 hours achieved an initial eradication rate of H. pylori of 95.3%
[3]. However, another study from China when optimizing high
dose amoxicillin and proton pump inhibitors did not achieve high
H.pylori eradication rates [4]. The effectiveness of high-dose dual
regimens in the treatment of H. pylori infection for the first time
has not been reported in our country, so we implement this topic
with the goal:
(1) Determination of H. pylori eradication rate of high-dose
dual regimen (rabeprazole - amoxicillin) in patients with
gastric or duodenal / gastric ulcer.
(2) Determine patient compliance and side effects for highdose
dual regimens.
Materials and Methods
Patients diagnosed with H. pylori infection who were indicated for treatment at the gastroenterology clinic of Ho Chi Minh City University of Medicine and Pharmacy
Inclusion Criteria: Patients 18 years of age and older
diagnosed with H. pylori infection who were indicated for treatment
at the gastroenterology clinic of Ho Chi Minh City University of
Medicine and Pharmacy.
Patients diagnosed with H. pylori infection when positive urease
test (CLO test) was indicated for H. pylori treatment including
Exclusion Criteria:
1. Stomach / duodenal ulcer diagnosed by upper
gastrointestinal endoscopy
2. Functional dyspepsia according to ROME IV-2016
standards Exclusion criteria
3. Stomach cancer (diagnosed with endoscopy with or
without pathology)
4. Severe medical conditions: decompensated cirrhosis,
end-stage chronic kidney disease, severe heart failure, COPD...
5. Patient is using antibiotics other than those mentioned in
the above regimen
6. The patient has no prescription other than a prescription
for H. pylori infection for a period of 14 days
7. Pregnant or lactating women
All patients will have upper endoscopy and CLO test. Patients who were eligible for the study will be treated with high-dose double therapy for 14 days, without any combination of any other digestive drugs besides 2 drugs in the regimen such as probiotics, prokinetic drugs), antacid... with time, dosage as follows:
1. Rabeprazole 20mg (Pariet) 1 capsule x 4 times daily for
5.5 hours, 30 minutes to 60 minutes before meals.
2. Amoxicillin 500mg 2 tablets 4 times / day, every 5.5 hours
after a meal.
During treatment, all patients must eat 4 meals: breakfast, lunch, dinner and add a 4th appointment in the evening, the meals must have protein to stimulate gastric acid secretion. The patients were consulted about the effectiveness, how to take the medicine and the common side effects of the medicine. All prescriptions are carefully documented for how to take, possible side effects and re-examination dates, so that patients are not mistaken and less worried when experiencing side effects. Patients are given the phone number of the treating doctor to contact when needed. Patients were re-examined after 2-3 weeks to evaluate side effects and adherence. Check H. pylori status after 4-8 weeks of treatment with rapid urease test (CLO test) or C13 breath test (C13 ureabreath test). The patient did not take any other antibiotic or bismuth for at least 4 weeks, proton pump inhibitor for at least 2 weeks and H2 receptor antagonist for at least 1 week before rechecking for H.pylori infection. Evaluation of adherence: <50% of oral medication is not compliant, 50- <80% is poorly adhered, ≥80% is adherent. Patients who are ≥80% compliant will be placed in the analysis group according to the study design (PP) All data were analyzed using software of Stata 12, p <0.05 is statistically significant. Use the χ2 test or calibrate Yate’s to compare 2 ratios. Compare 2 averages by t tests.
Results
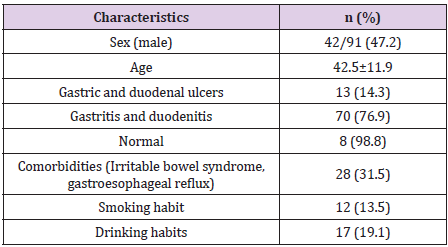
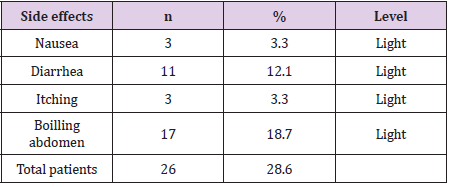
Most patients have endoscopy as gastritis, 9% normal endoscopy results diagnosed as functional indigestion (Table 1). There were 4 patients who adhered to the treatment <80%, 6 patients did not re-examine after the end of treatment 4-8 weeks, so the number of patients analyzed according to the study design was 82. High-dose dual therapy is highly effective in eradicating H. pylori (Table 2). The majority of patients had no side effects, the total number of patients with side effects was 28.6% and all side effects were mild (Table 3). Most of patients complied with ≥ 80% accounting for 94.5% (Table 4).
Discussion
The high dose double dose system used in this study was based
on two factors:
a) Low amoxicillin resistance worldwide. On the other
hand, the bactericidal effect of amoxicillin on H. pylori is thought
to be dependent on concentration over time (AUC), so higher doses
several times a day will have better results.
b) H. pylori becomes non-replicating state and does not
respond to antibiotics when stomach pH is 3-6. When the stomach
pH is increased to 6-8, the bacteria will turn to a new multiplication
state to respond to antibiotics [3].
Following the recommendations of the United States School of Gastroenterology (ACG 2017) [1], high-dose dual therapy consists of standard or double dose proton pump inhibitors administered three or four times daily in combination with 750 mg or amoxicillin. 1000 mg orally 3 or 4 times / day. We chose rabeprazole as PPI in our study because rabeprazole is not metabolized by the CYP2C19 pathway and is therefore not affected by polymorphism of this enzyme. In addition, rabeprazole is a 2nd generation PPI that inhibits strong acids. A stable level of acid suppression has been shown to be important in optimizing the effects of antibiotics such as amoxicillin, an acid-resistant antibiotic. Our study using 20 mg of rabeprazole in combination with 1000 mg of amoxicillin 4 times a day for 2 weeks showed that the eradication rates based on ITT and PP analysis were 80.2% and 90.2% lower than The study of Yang JC and colleagues in Taiwan on the group of patients treated with H. pylori infection for the first time reached 95.3% [5]. However, the eradication rates in our door study were significantly higher than in a recent pooled study of 473 H.pylori-infected patients who failed their first eradication at 81.3% [6]. The elimination effect in the pooled study is not high, probably due to the heterogeneity between these studies on the type of PPI used and the dose of PPI, amoxicillin.
Thus, the H.pylori eradication effect of this dual therapy is related to the type of PPI used, the dose of amoxicillin, and the number of daily doses. High-dose dual therapy has a frequency of using the drug 4 times a day at 5.5 hours, which is a difficult problem for patients. However, because all patients in our study were carefully consulted, the compliance rate was good, reaching 94.5%. At the end of the study, 6 patients did not follow up again in a total of 91 patients enrolled in the original study. We assessed side effects by asking patients for side effects during treatment. These side effects were assessed on four levels: mild, moderate, severe and very severe. We noted that 26 patients (28.6%) had side effects, but all these side effects were mild and transient, no patients reported severe side effects. The side effects of this therapy include: nausea, diarrhea, itching and intestinal boil, as noted by Kwack [7] and Yang JC [5]. The results of this study showed that the incidence of side effects of high-dose dual therapy was much lower than that of bismuth 4-drug regimen when the incidence of side effects of bismuth 4-drug regimen was recorded in the autistic children’s study. The wall in 2017 [8] is 80.5%. Other studies on high-dose dual therapy have shown that it is safe, well tolerated, and has side effects.
Conclusion
High-dose dual therapy containing rabeprazole and amoxicillin for 14 days achieved a high eradication rate in the treatment of H. pylori infection for the first time with a high compliance rate and few side effects.
References
- Chey William D, LeontiadisGrigorios I, Howden Colin W, Steven F Moss (2017) ACG clinical guideline: treatment of Helicobacter pylori infection. The American journal of gastroenterology 112(2): 212-239.
- Malfertheiner P, Megraud F, OMorain CA, JP Gisbert, EJ Kuiper, et al. (2017) Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 66(1): 6-30.
- Scott David R, Marcus Elizabeth A, Wen Yi, et al. (2007) Gene expression in vivo shows that Helicobacter pylori colonizes an acidic niche on the gastric surface. Proceedings of the National Academy of Sciences 104(17): 7235-7240.
- JiaLi Hu, Jun Yang, YinBin Zhou, Jane Oh, George Sachs (2017) Optimized high-dose amoxicillin–proton-pump inhibitor dual therapies fail to achieve high cure rates in China. Saudi J Gastroenterol 23(5): 275-280.
- Yang JyhChin, Lin ChunJung, Wang HongLong, Jin De Chen, John Y Kao, et al. (2015) High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clinical Gastroenterology and Hepatology 13(5): 895-905.
- Gao Cai Ping, Zhou Zhou, Wang Jia Zhen, Sheng Xi Han 1, Liang Ping Li, et al. (2016) Efficacy and safety of high‐dose dual therapy for Helicobacter pylori rescue therapy: A systematic review and meta‐analysis. Journal of digestive diseases 17(12): 811-819.
- KwackWonGun, Lim YunJeong, Lim ChiYeon, Graham David Y (2016) High Dose Ilaprazole/Amoxicillin as First-Line Regimen for Helicobacter pylori Infection in Korea. Gastroenterology research and practice: 1648047.
- Tran ThiKhanhTuong (2017) The efficacy of 4-medication protocol included Bismuth for intreatment Helicobacter Pylori infection. Vietnam Journal of Gastroenterology 49(9): 3067-3073.

 Research Article
Research Article