Abstract
Eggs of the marine medaka, Oryzias dancena were collected and fertilized to observe the temperature-salinity-related cleavage rates and mitotic intervals (0). We investigated the relationship between 0 and five different water temperatures (18, 22, 26, 30, and 34°C) and four different salinities (0, 10, 20, and 30 ppt NaCl). As the water temperature increased, the slope of the first cleavage frequency increased with elapsed time after fertilization, and approximately 30% of fertilized eggs reached the first cleavage frequency at every 15-minute intervals. However, the slope of the first cleavage frequency did not differ significantly between 0 ppt and the other salinities (10, 20, or 30 ppt). At higher water temperatures, the eggs developed more rapidly, but no other developmental process was affected. At higher salinity, the hatching rates of the eggs decreased, and the hatching times were delayed. There was a strong negative correlation between 0 and water temperature as shown in this equation (Y = –1.137X + 75.47, R2 = 0.977, where Y is 0 and X is water temperature). At a constant water temperature, 0 did not differ significantly in 0, 10, 20, and 30 ppt NaCl.
Keywords: Mitotic interval (0); Temperature-Salinity Dependence; Marine Medaka; Oryzias dancena
Short Communication
The marine medaka, Oryzias dancena is a euryhaline teleost that can live in both freshwater and seawater [1]. It spawns 60 days after hatching, with a consequently short interval between generations [2,3]. Accordingly, the species has drawn attention as an experimental animal in aquaculture. The marine medaka is more tolerant of hyperosmotic environments than is the Japanese medaka, O. latipes, with higher survival rates in the adult fish and greater hatching rates in the oosperm [3-6]. That is, most of its physiological attributes are similar across a wide spectrum of salinities, ranging from fresh water to normal seawater [4-6]. Therefore, much attention has been directed at extending the utility of functional transgenic marine medaka strains for ornamental purposes, because they can be used at most naturally occurring salinities [7]. Recently, marine medaka has been in the spotlight as an experimental fish of transgenic fish research [7-10]. However, the practical application of transgenic fish has raised public and scientific concern about the ecological risks involved, especially those associated with the adverse consequences for natural gene pools, which can be genetically contaminated if unwanted transgenic animals are released [11]. For these reasons, much recent scientific research has focused on risk assessment in relation to transgenic fish, with particular emphasis on the reproductive confinement of transgenic stocks [12]. Triploidization involving blocking of the second meiotic division has been proposed as one approach to the generation of transgenic fish having depressed reproductive capacities [8-10,13].
The ability to effectively manipulate ploidy through the application of suitable shocks (temperature, pressure, or chemical) early in egg development requires the empirical determination of the magnitude, duration, and time that the shock should be applied [8-10,14,15]. The genetic material of a gynogenetic haploid organism can be doubled by controlling the second meiotic division and the first cleavage. The second meiotic division can be controlled by water temperature, hydrostatic pressure, or chemical treatment, which can also be used to induce triploidy [15,16]. The first cleavage can be controlled by heat shock or hypostatic pressure, which are also used individually or sequentially to induce tetraploidy [17- 20].
The effectively controlled release of the second ootid and the first cleavage are dependent on the type, intensity, and duration of the treatment [21-23]. The method of controlling the first cleavage as a means of chromosomal engineering can also enhance shortterm production in aquaculture [14]. It also has an application in the induction of tetraploidy, mitotic gynogenetic diploidy, or androgenetic diploidy using chromosomal engineering. To effectively control the first cleavage, an understanding of its temperature dependence is essential [14,24]. However, determination of mitotic interval is necessary for producing triploid and tetraploid. The Dettlaff unit (mitotic interval, 0) is the duration (in minute) of one mitotic cycle during the early synchronous embryonic cleavage or the interval between two consecutive cell divisions [18,20,25,26]. When measured over a range of water temperatures, the relationship between 0 and water temperature, as determined by regression analysis, can showthe developmental events that are influenced by temperature within a species and between species with a similar spawning biology [18,20,27].
Up to date, 0 has been used to estimate the optimal times for chromosomal manipulation in a variety of species, including the tench, Tinca tinca; common carp; Cyprinus carpio; paddlefish, Polyodon spathula; shovelnose sturgeon, Scaphirhynchus platorynchus; black crappie, Pomoxis nigromaculatus; far eastern catfish, Silurus asotus; winter flounder, Pseudopleuronectes americanus; greenling, Hexagrammos otakii; black plaice, Pleuronectes obscurus; and Korean rose bitterling, Rhodeus uyekii [26,28-36]. Because the marine medaka has a wide range of salinity tolerance, we identified the egg development achieved through artificial fertilization, and also assessed the temperature–salinity dependence of 0 and the embryo cleavage rates to establish the most efficient procedures for chromosome manipulation in the marine medaka.
Materials and Methods
The experimental group of diploid marine medaka, Oryzias dancena used in this study was reared according to the methods of the previous study [37]. Fish with a standard length of over 25 mm were used in this experiment; 35 males and 15 females were placed in each of two aquariums, and 2,000 fertilized eggs were collected immediately by net. The fertilized eggs of the experimental group (n = 100) were reared in a 100 L glass aquarium. To assess the temperature-salinity dependence of the first cleavage and 0, the water temperature was maintained in temperature-controlled water baths at 18, 22, 26, 30, or 34°C, and each experimental group was subjected to one of four salinities, 0, 10, 20, or 30 ppt NaCl. The water salinity of each group was regulated to remain constant throughout the experiment. Samples were generally taken at 5-minute intervals and fixed with 5% neutral formalin solution (50 mL formalin, 3.25 g Na2HPO4·12H2O, 2.25 g KH2PO4, 950 mL distilled water) at 4°C before observation. We measured the diameter of 10 fertilized eggs at a magnification of 50 under an optical microscope (Axiostar plus, Zeiss, Germany) equipped with a microscope camera (Axiocam MR, Zeiss). A specific developmental stage was considered to be reached when approximately 80% of the developing embryos were at that stage. In this study, all experiments were performed in triplicate.
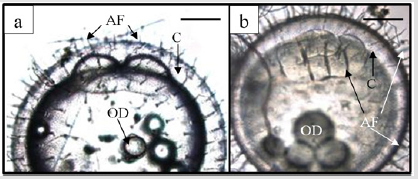
The time of the appearance of the first cleavage furrow was recorded and was deemed to be the start of the subsequent cell division. The times at which approximately 10% of the developing embryos reached the two-cell (I; Figure 1a) and eight-cell (III; Figure 1b) stages were recorded. The value of 10% was selected according to the recommendation of the previous study [38]. The mean 0 value was calculated as 0 = (III – I)/2. The relationship between the mean 0 and the water temperature was examined by simple linear regression line. One-way and two-way ANOVA analysis were applied to determine water temperature and salinity effects. Duncun’s multiple range test was applied when significant difference was observed.
Figure 1: External morphology of egg development in marine medaka, Oryzias dancena. (a): 2-celled stage; (b): 8-celled stage. Scale bars are 100 μm. AF: attaching filament; C: chorion; OD: oil droplet.
Result
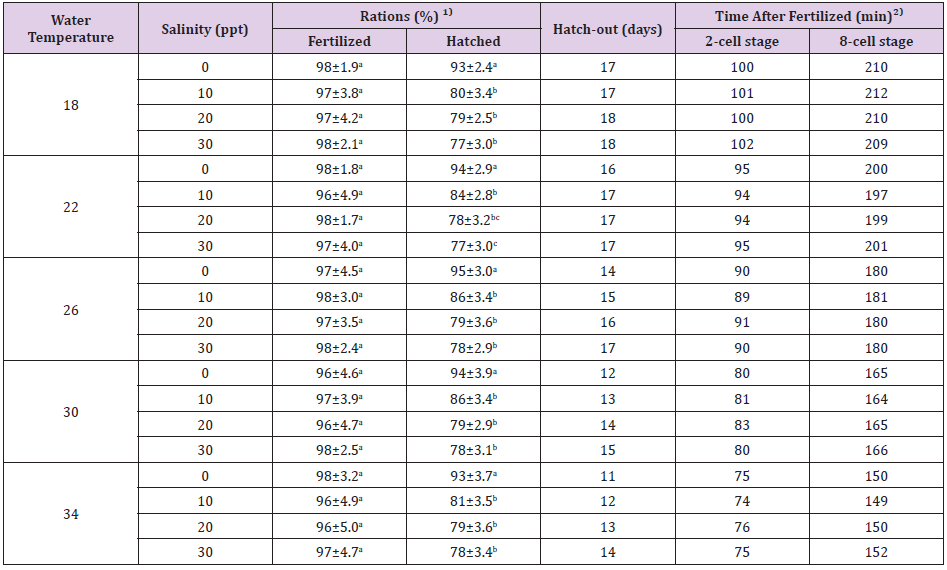
The fertilization, hatching rates, and hatching times of the marine medaka, Oryzias dancena eggs are shown in Table 1. The fertilization rates of the groups were not significantly different, and the hatching rates of each group did not differ significantly as the water temperature increased. With increasing water salinity, the hatching rates decreased at each specific water temperature, but the hatching rates of each group did not differ significantly at a specific salinity respectively. With increasing water salinity, the hatching time of each group was delayed in each specific water temperature and its time was delayed as the water temperature decreased in each specific salinity respectively. As water temperature increased, the two-cell stage and the eight-cell stage were reached earlier in a specific salinity. However, the times taken to reach the two-cell stage and eight-cell stage did not differ across different salinities at the same water temperature (Table 1).
Table 1: Fertilized and hatched rates, and 2 cell stage, 8 cell stage and hatch-out time of each group in marine medaka, Oryzias dancena. 1)Each value (means of triplicate ± SD) having different superscript letters are significantly different (P<0.05). Fertilized rates of each group were analyzed at 24 hrs after fertilized. Hatched rates of each group were analyzed at 1 day after hatch-out. 2)Each time were analyzed when 80% of total eggs were reached.
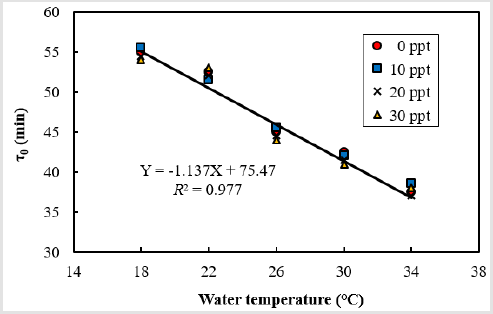
Figure 2: Mitotic intervals (τ0, Y) for marine medaka, Oryzias dancena as functions of temperature (X). Temperatures used are within the normal range for spawning and early, development in this species. Eggs from three females to were fertilized with pooled sperm from one male and were distributed between the temperature treatments and the salinity treatments. The experiments were executed three times.
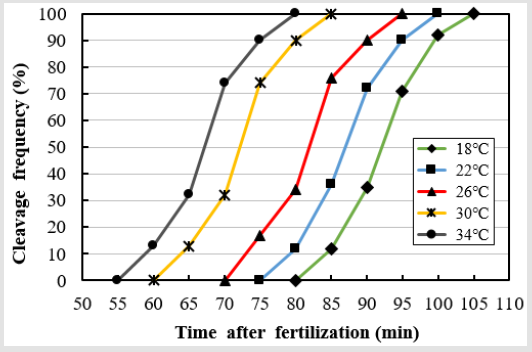
As shown in Figure 2, the 0 values at 18, 22, 26, 30, and 34°C were 55, 52.5, 45, 42.5, and 37.5 minutes respectively, and there was a strong negative correlation between 0 and water temperature at all water temperatures (Y = –1.137X + 75.47, R2 = 0.977, where Y is 0 and X is water temperature). Within the groups subjected to the same water temperature, 0 did not differ at salinities of 0, 10, 20, and 30 ppt NaCl. As shown in Figure 3, the eggs of the marine medaka at a salinity of 0 ppt showed the fastest development at higher water temperatures. The first cleavage began after 80, 75, 70, 60, and 55 minutes at the water temperatures of 18, 22, 20, 30, and 34°C respectively. The embryos reached the two-cell stage after 100, 95, 90, 80, and 75 minutes at the water temperatures of 18, 22, 26, 30, and 34°C respectively. As the water temperature increased, the slope of the first cleavage frequency with elapsed time after fertilization increased, and approximately 30% of the fertilized eggs reached the first cleavage frequency at every 5-10 minutes intervals. However, the slope of the first cleavage frequency with time did not differ significantly between a salinity of 0 ppt and the other salinities (10, 20, and 30 ppt NaCl).
Figure 3: The percentages of marine medaka, Oryzias dancena eggs developed to anaphase of first cleavage at 0 ppt salinity and five different temperatures overtime.
Discussion
The previous study has reported the embryogenesis and early ontogenesis of the marine medaka, Oryzias dancena [2-3]. As they mentioned, the marine medaka eggs reaches the two-cell stage 90 minutes after fertilization at 26°C and 1 ppt NaCl and reaches the eight-cell stage 3 hrs after fertilization under these conditions. Although the experimental salinities used in our study differed from that of the previous study, the times required to reach the two-cell stage and eight-cell stage at 26°C and 0 ppt NaCl in our study were similar to the previous study at 26°C and 1 ppt NaCl [2]. Another previous study also reported the embryonic development and early viability of the marine medaka under different salinity conditions [38]. They reported that the hatching rates of their eggs decreased at higher salinities, and the hatching rate of the eggs was highest at 0 ppt NaCl among the salinities tested [38]. At higher salinities, the hatching times of the eggs were delayed, and its hatching time at 0 ppt NaCl was earliest among the experimental groups. In the salinity range of 0 ppt to 30 ppt NaCl, the hatching rates and hatching times in our study were similar to those of the previous study [38].
We also attempted to determine the water temperature-salinity dependence of the cleavage rates and 0 and found that the marine medaka eggs underwent cleavage within a temperature range of 18-34°C and a salinity range of 0-30 ppt NaCl. We found that the marine medaka eggs showed faster development with increasing water temperatures and water salinity, whereas 0 decreased with increasing water temperatures, which indicates a strong negative correlation between 0 and water temperature. Although 0 and hatching time after fertilization differed greatly from those of other species, the trend in the water temperature dependence of 0 for the marine medaka is similar to that in the black plaice, Pleuronectes obscurus; winter flounder, Pseudopleuronectes americanus; far eastern catfish, Silurus asotus; greenling, Hexagrammos otakii; Baltic herring, Clupea harengus membras; perch, Perca fluviatilis; ruffe, Gymnocephalus cernuus; Korean rose bitterling, Rhodeus uyekii; Korean bullhead, Pseudobagrus fulvidraco; grass puffer, Takifugu niphobles [18,20,25,32-36,39].
The relationship between 0 and water temperature in fish is typically curvilinear, when the temperatures are within the range in which the fish species naturally spawn and develop [18,20,25,32- 35,39]. The linear response we observed in this study for 0 against water temperature is consistent with studies of developmental rates in the black carp, the winter flounder, and the black plaice [18,20,29,33,35]. However, additional observations are required. The available data suggest that the graphs of the dependence of 0 on the water temperature are highly species specific. Therefore, the species specificity of the developmental rate can be used to identify the taxonomic ranges of different fish species. We searched the previous study for determining the relationship between 0 and the water salinity. Unfortunately, no previous studies have reported such a relationship between 0 and the water salinity. That’s because 0 of several species (having a wide spectrum of salinities) have not been researched so far. So, the future investigation of species having a wide spectrum of salinities needs to focus on the relationship between development and water salinity. The results of this study and an investigation of seeding production techniques based on artificial fertilization, the induction of triploidy to produce sterile organisms, and the induction of tetraploidy, mitotic gynogenetic diploidy, and androgenetic diploidy in the marine medaka will facilitate such research in the future as well.
Conclusion
Considering the identity of the mitotic events and the short time intervals of mitotic intervals (0), chromosomal manipulation in marine medaka, Oryzias dancena would be the most effective at 0 ppt salinity and water temperatures between 26 and 30°C. This study has demonstrated the specific and clear differences in the rates of hatching, the time of the first cleavage, τ0, and hatching time at different water temperatures and different salinities in the marine medaka. These data will be useful for the development of an optimal treatment protocol for its chromosomal manipulation. These data on eggs development will make a valuable contribution to a biological aquaculture database.
Declaration
Ethics Approval and Consent to Participate
The experiments performed in this study complied with the current laws of Korea (Ordinance of Agriculture, Food and Fisheries, No. 1 - the law regarding experimental animals, No. 9982) and the Ethical Guidelines of Korea Maritime & Ocean University, Korea.
Acknowledgement
I am grateful to the staff of the Fishery Genetics and Breeding Sciences Laboratory of the Korea Maritime & Ocean University, Korea. The author would like to thank anonymous reviewers for their helpful suggestions that improved the quality of this paper.
Conflict of Interest
The author has no financial or personal conflicts of interests.
References
- Roberts TR (1998) Systematic observations on tropical Asian medakas or rice fishes of genus Oryzias, with descriptions of four new species. Ichthyol Res 45: 213-224.
- Kim DS, Nam YK, Bang IC, Song HY (2009) Embryogenesis and early ontogenesis of a marine medaka (Oryzias dancena). Korean J Ichthyol 21: 227-238.
- Im JH, Gil HW, Lee TH, Kong HJ, Ahn CM, et al. (2016) Morphometic characteristics and fin dimorphism between male and female on the marine medaka, Oryzias dancena. Dev Reprod 20: 331-347.
- Inoue K, Takei Y (2003) Asian medaka fishes offer new models for studying mechanisms of seawater adaptation. Comp Biochem Physiol B 136: 635-645.
- Kang CK, Tsai SC, Lee TH, Hwang PP (2008) Differential expression of branchial Na+/K+-ATPase of two medaka species (Oryzias latipes) and (Oryzias dancena) with different salinity tolerances acclimated to fresh water, brackish water and seawater. Comp Biochem Physiol A 151: 566-575.
- Nam YK, Cho YS, Lee SY, Kim DS (2010a) Tolerance capacity to salinity changes in adult and larva of Oryzias dancena, a euryhaline medaka. Korean J Ichthyol 22: 9-16.
- Cho YS, Lee SY, Kim YK, Kim DS, Nam YK (2011) Functional ability of cytoskeletal β-actin regulator to drive constitutive and ubiquitous expression of a fluorescent reporter throughout the life cycle of transgenic marine medaka, Oryzias dancena. Trans Res 20: 1333-1355.
- Park I-S, Gil HW, Lee TH, Nam YK, Kim DS (2016b) Comparative study of growth and gonad maturation in diploid and triploid marine medaka, Oryzias dancena. Dev Reprod 20: 305-314.
- Park I-S, Gil HW (2018a) Comparative analysis of fluctuating asymmetry between ploidy and sex in marine medaka, Oryzias dancena. Dev Reprod 22: 275-281.
- Park I-S, Gil HW, Kim DS (2018b) Morphometric characteristics of diploid and triploid marine medaka, Oryzias dancena. Dev Reprod 22: 183-192.
- Devlin RH, Biagi CA, Yesaki TY (2004) Growth, viability and genetic characteristics of GH transgenic coho salmon strains. Aquaculture 236: 607-632.
- Hu SY, Lin PY, Liao CH, Gong HY, Lin GH, et al. (2010) Nitroreductase-mediated gonadal dysgenesis for infertility control of genetically modified zebrafish. Mar Biotechnol 12: 569-578.
- Piferrer F, Beaumont A, Falguière JC, Flajšhans M, Haffray P, et al. (2009) Polyploid fish and shellfish: Production, biology and applications to aquaculture for performance improvement and genetic containment. Aquaculture 293: 125-156.
- Thorgaard GH (1983) Chromosome set manipulation and sex control in fish. In Hoar WS, Randall DT, Donaldson EM, (Eds.), Fish physiology Vol 9B. Academic Press, New York, USA, pp: 405-434.
- Goo IB, Im JH, Gil HW, Lim SG, Park I-S (2015) Comparison of cell andnuclear size difference between diploid and induced triploid in marine medaka, Oryzias dancena. Dev Reprod 19: 127-134.
- Park I-S, Gil HW, Lee TH, Nam YK, Ko MG, et al. (2016a) Cytogenetic study of diploid and triploid marine medaka, Oryzias dancena. Korean J Ichthyol 28: 215-222.
- Komen J, Bongers ABJ, Richter CJJ, Muiswinkel V, Huisman EA (1991) Gynogenesis in common carp (Cyprinus carpio). Ⅱ, The production of homozyous gynogenetic clones and F1 hybrids. Aquaculture 92: 127-142.
- Lim S-G, Han HK, Gil HW, Park I-S (2012) Temperature-dependent index of mitotic interval (t0) for chromosome manipulation in Korean bullhead, Pseudobagrus fulvidraco. Dev Reprod 16: 321-327.
- Gil HW, Kong HJ, Ahn CM, Kim B-S, Lim SG, Park IS (2016) Cytogenetic study of diploid and induced tetraploid in Korean rose bitterling, Rhodeus uyekii. Springer Plus 5: 186-195.
- Ko MG, Lee HB, Gil HW, Kang SB, Park IS, et al. (2018) Temperature dependent of mitotic interval for grass puffer, Takifugu niphobles. Dev Reprod 22: 111-117.
- Thorgaard GH, Jazwin ME, Stier AR (1981) Polyploidy induced by heat shock in rainbow trout. Trans Amer Fisher Soc 110: 546-550.
- Onozato H, Yamaha E (1983) Induction of gynogenesis with ultraviolet rays in four species of Salmoniforms. Bull Jpn Soc Sci Fish 49: 693-699.
- Thorgaard GH, Allen SK Jr (1987) Chromosome manipulation and markers in fishery management. In Ryman N, Utter FM, (Eds.), Population Genetics and fisheries management, Univ Washington Press, Seattle, USA, pp. 319-331.
- Mair GC (1993) Chromosome-set manipulation in tilapia - techniques, problems and prospects. Aquaculture 111: 227-244.
- Saat T, Veersalu A (1996a) Duration of synchronous cleavage cycles and rate of development at different temperatures in the Baltic herring. J Fish Biol 48: 658-663.
- Shelton WL, Mims SD, Clark JA, Hiott AE, Wang C (1997) A temperature-dependent index of mitotic interval (τ0) for chromosome manipulation in paddlefish and shovelnose sturgeon. Prog Fish-Cultur 59: 229-234.
- Dettlaff TA (1986) The rate of development in poikilothermic animals calculated in astronomical and relative time units. J Therm Biol 11: 1-7.
- Flajšhans M, Linhart O, Kvasnika P (1993) Genetic studies of tench (Tinca tinca L.): induced triploidy and tetraploidy and first performance data. Aquaculture 113: 301-312.
- Shelton WL, Rothbard S (1993) Determination of the developmental duration (τ0) for ploidy manipulation in carps. Israeli J Aquacult - Bamidgeh 45: 73-81.
- Mins SD, Shelton WL, Linhart O, Wang C (1997) Induced meiotic gynogenesis of paddlefish Polyodon spathula. J World Aquacult Soc 28: 334-343.
- Gomelsky BI, Mims SD, Onders RJ, Shelton WL, Dabrowski K, et al. (2000) Induced gynogenesis in black crappie. North Amer J Aquacult 62: 33-41.
- Park I-S, Im JH (2001) Determination of the temperature-dependent index of mitotic interval (τ0) for chromosome manipulation in far eastern catfish, Silurus asotus. Korean J Ichthyol 13: 85-88.
- Park I-S, Johnson SC (2002) Determination of the temperature-dependent index of mitotic interval (τ0) for chromosome manipulation in winter flounder Pseudopleuronectes americanus. Aquaculture 213: 95-100.
- Park I-S, Kim E-M, Woo, SR, Oh S-Y, Kim DS, et al. (2006) Temperature-dependent index of mitotic interval τ0 in greenling Hexagrammos otakii. Fish Sci 72: 719-722.
- Park I-S, Im S-Y (2010) Egg development and mitotic interval in black plaice, Pleuronectes obscurus (Herzenstein). Fish Aquacult Sci 13: 278-283.
- Park I-S, Kim B-S, Lim S-G, Gil HW (2011b) Temperature-dependent index of mitotic interval (τ0) for chromosome manipulation in Korean rose bitterling, Rhodeus uyekii. Fish Aquacult Sci 14: 1-5.
- Park I-S, Park SJ, Gil HW, Nam YK, Kim DS (2011a) Anesthetic effects of clove oil and lidocaine-HCl on marine medak, Oryzias dancena. Lab Animal 40: 45-51.
- Nam YK, Cho YS, Lee SY, Kim DS (2010b) Spawning performance, embryonic development and early viability under different salinity conditions in a euryhaline medaka species, Oryzias dancena. Korean J Ichthyol 22: 25-33.
- Saat T, Veersalu A (1996b) The rate of early development in perch Perca fluviatilis L. and ruffe Gymnocephalus cernuus (L.) at different temperatures. Annales Zoologici Fennici 33: 693-698.

 Short Communication
Short Communication