Abstract
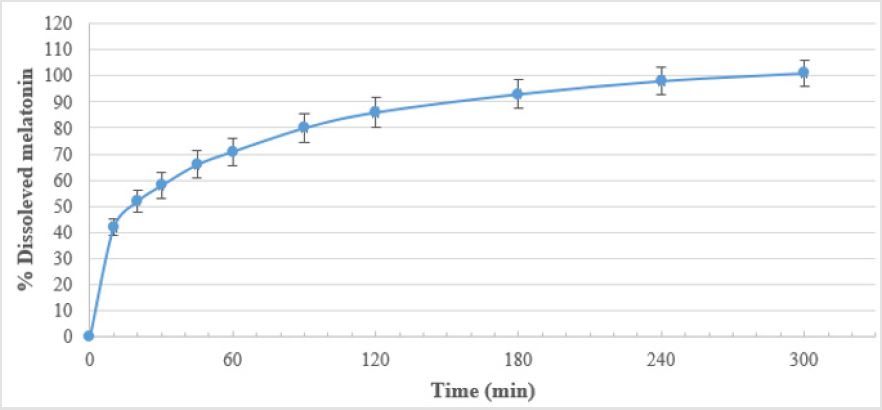
Oniria® is a food supplement containing 1.98 mg of melatonin per tablet. To study its dissolution profile, an in vitro dissolution test has been performed in which we have demonstrated the prolonged-release of the melatonin tablets and a characteristic dual dissolution profile (an initial burst of approximately 50% of tablet in the first 20 minutes, followed by 50% remaining up to 5 hours).
Keywords: Melatonin; Delayed-Action Preparations; Drug Liberation
Introduction
Melatonin is an endogenous substance which is decisively involved in the physiological control of the circadian sleep-wake cycle [1]. Its exogenous administration as food supplement has been assessed and approved by the EFSA (English acronym of European Food Safety Agency) for the reduction of sleep latency, thus favoring induction of sleep [2]. Prolonged-release forms of melatonin are known to be more slowly and sustainably absorbed than the immediate release ones; in the case of melatonin this behavior could lead to pharmacokinetic (PK) patterns comparable to the physiological secretion of endogenous melatonin [3]. The in vitro dissolution characteristics of Oniria®, a food supplement containing 1.98 mg of melatonin per coated tablet, is described to show the prolonged-released properties of Oniria®.
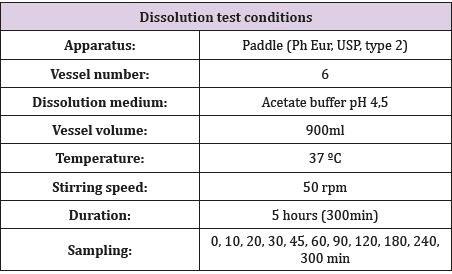
Material and Methods
Two assays with 6 vessels each one was carried out to obtain results from a total of 12 vessels. The dissolution bath was prepared under conditions similar to gastric (temperature 37 °C; pH 4.5). 6 Oniria® tablets (1.98 mg per tablet) were exactly weighed in an analytical scale and each one was placed in a glass of the dissolution bath. At the corresponding times (0, 10, 20, 30, 45, 60, 90, 120, 180, 240 and 300 min), and for a maximum of 5 hours, 10 ml were taken from each glass with the help of a syringe (see Table 1 for Dissolution test conditions). At each sampling time, the volume was replenished with the same volume as extracted with thermostated medium from an additional vessel prepared for this purpose. Melatonin quantification was performed using the HPLC (High Performance Liquid Chromatography) Agilent 1260 Infinity II HPLC equipment and Kinetex 2.6μm C8 100 x 4.6mm column.
Results
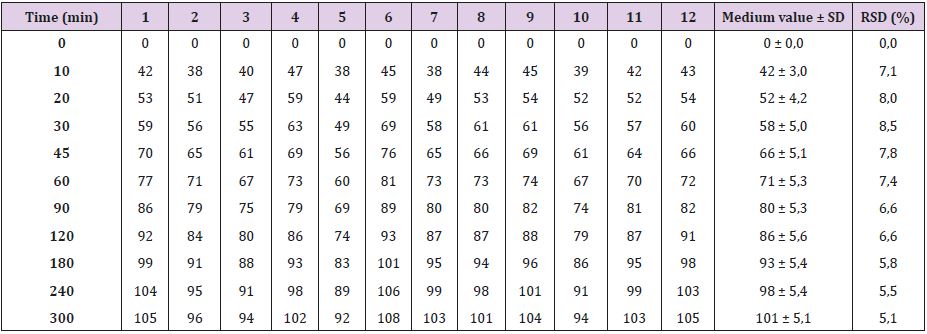
As seen in Table 2 and Figure 1, Oniria® has a dissolution profile in which the release of approximately 50% of the content of melatonin (1 mg) occurs before the first 20 minutes. The release of the rest of melatonin active ingredient is prolonged for up to 300 minutes (5 hours). Therefore, Oniria® is a prolonged-release product with a dissolution profile composed of an initial phase when about half of the product is rapidly released, followed by a subsequent slower release phase of the other half.
Table 2: Percentage of dissolved melatonin at each sampling time.
SD: Standard Deviation; RSD: Relative Standard Deviation
Conclusion
As for our findings from the reported dissolution test, Oniria® is a food supplement product containing 1.98 mg melatonin prolongedrelease tablets, with a dual dissolution profile in which about 1mg (50%) is released in the first 20 minutes, and the remaining 50% up to 5 hours. Therefore, and as endorsed by EFSA, it can reduce the sleep latency and promote the induction of sleep.
Conflicts of Interests
Martínez V, and González J are employees of ITF Research Pharma, S.L.U., Alcobendas, Madrid, Spain.
References
- Geoffriau M, Brun J, Chazot G, Claustrat B (1998) The physiology and pharmacology of melatonin in humans. Horm Res 49(3-4): 136-141.
- (2011) EFSA (European Food Safety Authority) Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to melatonin and reduction of sleep onset latency (ID 1698, 1780, 4080) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 9(6): 2241.
- Poza JJ, Pujol M, Ortega Albás JJ, Romero O (2018) on behalf of the Insomnia Study Group of the Spanish Sleep Society (SES). Melatonin in sleep disorders. Neurologia pii: S0213-4853(18): 30200-30207.

 Short Communication
Short Communication