Research Article
Effect of Sacubitril/Valsartan on VEGF, VEGFR-1 and
Left Ventricular Remodeling in Patients with Heart
Failure with Decreased Ejection Fraction
Xia Han*1, Menghai Wu2, Huiqin Qi1 and Wenting Liu1
Author Affiliations
1Department of Cardiology, Jinan People’s Hospital Affiliated to Shandong First Medical University, China
2Department of Neurology, Jinan People’s Hospital Affiliated to Shandong First Medical University, China
Received: January 30, 2020 | Published: February 10, 2020
Corresponding author: Xia Han, Department of Cardiology, Jinan People’s Hospital Affiliated to Shandong First Medical
University, Laiwu 271199, China
DOI: 10.26717/BJSTR.2020.25.004203
Background: Sacubitril/valsartan has been shown to reduce mortality and reduce
hospitalization in patients with heart failure with reduced ejection fraction (HFrEF),
but it has not been reported for endothelial function and left ventricular remodeling in
patients with heart failure. Endothelial cell dysfunction is involved in the progression of
heart failure.
Hypothesis: So, we hypothesized that Sacubitril/valsartan improves left ventricular
remodeling and prognosis in patients with heart failure by affecting VEGF and VEGFR-1.
Methods: From October 2018 to May 2019, 63 patients with heart failure who
had been hospitalized in the Department of Cardiology of Jinan People’s Hospital
were selected. According to the treatment plan, they were divided into control group
(37 cases) and observation group (26 cases). Patients in the control group underwent
routine anti-heart failure treatment. Patients in the observation group were replaced
with angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor antagonist
(ARB) in the conventional anti-heart failure treatment regimen. Valsartan; both groups of
patients were treated for 3 months. The levels of NT-proBNP, VEGF, VEGFR-1 and cardiac
structural parameters were compared before and after treatment in the two groups.
Results: There were significant differences in VEGF, VEGFR-1 and pro-BNP between
the two groups after T treatment.
Conclusion: After 3 months of treatment with Sacubitril/valsartan, patients with
heart failure with reduced ejection fraction improved left ventricular systolic function,
increased plasma VEGF levels, and decreased NT-proBNP and VEGF-1R levels.
Keywords:Myocardial Infarction; Heart
Failure; Sacubitril/Valsartan; VEGF;
VEGFR-1
Percutaneous Coronary Intervention (PCI) completely altered
the management and treatment of acute Myocardial Infarction (MI)
[1,2]. It is a reperfusion strategy for the entire developed country,
with only 90,000 operations performed annually in the United
States [3,4]. The introduction of PCI and adjuvant therapy have
reduced hospital mortality after acute Myocardial Infarction from
20% in the late 1980s to about 5%-7% in the modern series [5,6].
Heart failure after Myocardial Infarction remains a major driver
of coronary heart disease in patients with advanced morbidity,
mortality, and medical costs. There is therefore an urgent need to
explore new therapies for patients with reduced ejection fraction.
A new type of drug for the treatment of heart failure, Sacubitril/
valsartan, has been developed, a salt complex crystal composed of
Using an open-ended test, the observation group and the control
group were randomly divided according to the random number
table method.
Patients in the control group were treated with conventional
anti-heart failure, including rest, salt restriction and diuretics,
digitalis preparations, vasodilators, beta-blockers, Angiotensin-
Converting Enzyme Inhibitors (ACEI)/angiotens Receptor II receptor antagonist (ARB), spironolactone treatment, etc., and
adjust the treatment according to the primary disease; patients in
the observation group replaced the ACEI/ARB in the conventional
anti-heart failure treatment plan with Sacubitril/valsartan (Beijing)
Novartis Pharmaceutical Co., Ltd. produces, Chinese medicine
quasi-word H20170344), starting dose 25 mg / time, 2 times / d,
then gradually increase the dose, the maximum dose is 400 mg / d,
in order to overlap the risk of angioedema caused by ACEI To the
lowest, before the start of the use of Using an open-ended test, the
observation group and the control group were randomly divided
according to the random number table method.
Patients in the control group were treated with conventional
anti-heart failure, including rest, salt restriction and diuretics,
digitalis preparations, vasodilators, beta-blockers, Angiotensin-
Converting Enzyme Inhibitors (ACEI)/Angiotens Receptor II
Receptor Antagonist (ARB), spironolactone treatment, etc., and
adjust the treatment according to the primary disease; patients in
the observation group replaced the ACEI/ARB in the conventional
anti-heart failure treatment plan with Sacubitril/valsartan (Beijing)
Novartis Pharmaceutical Co., Ltd. produces, Chinese medicine
quasi-word H20170344), starting dose 25 mg / time, 2 times / d,
then gradually increase the dose, the maximum dose is 400 mg /
d, in order to overlap the risk of angioedema caused by ACEI To
the lowest, before the start of the use of Sacubitril/valsartan
ACEI should be given at least 36 h for drug elution, the rest of the
treatment with the control group. Both groups of patients were
treated continuously for 3 months.
Combination therapy with ACE inhibitor (or ARB) and
Sacubitril/valsartan is strictly prohibited. Two groups of patients
underwent routine clinical examination before treatment with
anti-heart failure, 12-lead electrocardiogram (ECG), transthoracic
echocardiography (TTE) and Doppler assessment. The same
measurement was repeated at 3 months. Cardiac function
assessment was performed using NYHA classification. / valsartan,
ACEI should be given at least 36 h for drug elution, the rest of
the treatment with the control group. Both groups of patients
were treated continuously for 3 months. Combination therapy
with ACE inhibitor (or ARB) and Sacubitril/valsartan is strictly
prohibited. Two groups of patients underwent routine clinical
examination before treatment with anti-heart failure, 12-lead
electrocardiogram (ECG), transthoracic echocardiography (TTE)
and Doppler assessment. The same measurement was repeated
at 3 months. Cardiac function assessment was performed using
NYHA classification.and valsartan (LCZ696) in a 1:1 molar ratio
[7] , PARADIGM-HF [ARNI vs. ACEI (a prospective comparison
of angiotensin-converting enzyme inhibition to determine the
overall mortality and morbidity of heart failure) study] is a phase
III, randomized, double-blind trial evaluation. The safety and
efficacy [8] of LCZ 696 and enalapril in patients with chronic
symptomatic HFREF can reduce all-cause mortality in patients with
chronic HFREF by 16%, cardiovascular mortality by 20%, and HF hospitalization by 21%. However, in this landmark trial, the serum
markers VEGF, VEGFR-1 and endothelial cell-controlled signaling
pathways that do not clearly determine. Endothelial cell-controlled
signaling pathways play a crucial homeostatic role in cardiac tissue
and disorders of these pathways can lead to poor myocardial
remodeling and dysfunction in heart failure, suggesting a poor
prognosis in these patients.
Vascular Endothelial Growth Factor (VEGF) is a plateletderived
growth factor supergene family secreted by Endothelial
Cells and plays a central role in regulating angiogenesis and
lymphangiogenesis. VEGF-A is a major angiogenic factor that
binds to two Tyrosine Kinase (TK) receptors, VEGFR-1 (Flt-1) and
VEGFR-2 (KDR/Flk-1), and regulates proliferation of endothelial
cells. , migration, vascular permeability, secretion and other small
molecule proteins or peptides. Our study aimed to evaluate the
effect of Sacubitril/valsartan on endothelial cell function (VEGF/
VEGFR-1) and to further provide new therapeutic targets and
clinical markers for heart failure.
The study was approved by our Institutional Committee on
Human Research. No extramural funding was employed to support
this work. The authors are solely responsible for study design and
conduct, study analyses, drafting and editing of the paper, as well as
its final editorial content.
Patient Selection
From June 2017 to September 2018, patients with heart
failure who had a reduced ejection fraction in the Department of
Cardiology of Jinan City People’s Hospital.
Standard Constrain: The patient is at least 18 years of age,
with New York Heart Association (NYHA) II, III or IV symptoms and
an ejection fraction of no more than 40%. The patient was asked
to be hospitalized for heart failure in the past 12 months. Consider
patients who do not take any ACE inhibitors or ARB, or who take a
steady dose of beta blockers, ACE inhibitors or ARB for at least 4
weeks.
Exclusion Criteria: Including symptomatic hypotension,
systolic blood pressure <100 mmHg, estimated glomerular
filtration rate (eGFR) below body surface area 30 mL / min /
1.73 m2, serum potassium level > 5.2 mmol / L during screening,
history of angioedema Or unacceptable side effects occur with
ACE inhibitors or ARB treatment. Other exclusion criteria were as
follows: correctable valvular disease; <3 months of acute coronary
syndrome; recent coronary revascularization within the last 3
months, or planned revascularization. According to previous
studies, an (absolute) improvement in LVEF ≥ 5% is considered to
be an important response to shakuba/valsartan [9 ,10].
Study Procedures
Using an open-ended test, the observation group and the
control group were randomly divided according to the random number table method. Patients in the control group were treated
with conventional anti-heart failure, including rest, salt restriction
and diuretics, digitalis preparations, vasodilators, beta-blockers,
Angiotensin-Converting Enzyme Inhibitors (ACEI)/Angiotens
Receptor II Receptor Antagonist (ARB), spironolactone treatment,
etc., and adjust the treatment according to the primary disease;
patients in the observation group replaced the ACEI/ARB in the
conventional anti-heart failure treatment plan with Shakuba /
valsartan (Beijing Novartis Pharmaceutical Co., Ltd. produces,
Chinese medicine quasi-word H20170344), starting dose 25 mg /
time, 2 times / d, then gradually increase the dose, the maximum
dose is 400 mg / d, in order to overlap the risk of angioedema caused
by ACEI To the lowest, before the start of the use of Sacubitril/
valsartan, ACEI should be given at least 36 h for drug elution, the
rest of the treatment with the control group. Both groups of patients
were treated continuously for 3 months. Combination therapy
with ACE inhibitor (or ARB) and Sacubitril/valsartan/valsartan
is strictly prohibited. Two groups of patients underwent routine
clinical examination before treatment with anti-heart failure, 12-
lead electrocardiogram (ECG), transthoracic echocardiography
(TTE) and Doppler assessment. The same measurement was
repeated at 3 months. Cardiac function assessment was performed
using NYHA classification.
Echocardiographic Measurements
Vivid E9, GE Healthcare, (USA) was used to detect patients
taking the left lateral position and synchronizing the ECG. Subjects
underwent routine cardiac ultrasound by an experienced cardiac
sonographer (without knowing the patient’s condition), using an s3
ultrasound probe with a probe frequency of 2.5 MH and measuring
left ventricular structural parameters such as end-systolic and
diastolic at a standard plane recommended by the American
Society of Ultrasound. End diameter (LVIDs, LVIDd), posterior
wall end-systolic and end-diastolic thickness (LVPwTs, LVPWTd),
systolic and end-diastolic volume (LVESV, LVEDV), systolic function
(LVEF), diastolic function (E/A, EDT, IVRT) Routine indicators, left
and right ventricle and left and right ventricle (LA, LV) in the apical
four-chamber view. All indicators were measured continuously for
3 cycles. All echocardiograms are read, reviewed, and diagnosed
by trained experts. LVEF: Left ventricular end-diastolic volume
(LVEDV), left ventricular end-systolic volume (LVESV), and left
ventricular ejection fraction (LVEF) were measured using the
Simpson method. LVEF = (LVEDV - LVESV) / LVEDV × 100%.
Detection of VEGF and VEGFR-1
The kit uses a double antibody one-step sandwich enzymelinked
immunosorbent assay (ELISA). The coated microcapsules,
which are pre-coated with Vascular Endothelial Growth Factor
(VEGF) antibody and Vascular Endothelial Growth Factor receptor
1 (VEGFR-1) antibody, are sequentially added with specimens,
standards, and HRP-labeled detection antibodies. Breed and wash
thoroughly. Using the substrate TMB to develop color, TMB is
converted to blue under the catalysis of peroxidase and converted to the final yellow color by the action of an acid. The color depth is
positively correlated with VEGF and VEGFR-1 in the sample. The
absorbance (OD value) was measured at 450 nm using a microplate
reader to calculate the sample concentration.
Statistical Analysis
Data analysis was performed using SPSS 13.0 statistical
software. The measurement data were expressed as mean ± SD. The
t test was used for comparison between groups. The paired t test
was used for comparison within the group. The χ2 test was used for
the analysis of the count data test. The difference was statistically
significant at P < 0.05. The skewed distribution data is converted to
a normal distribution (represented by the median) by log logarithm.
Population
All patients were divided into control group (37 cases) and
observation group (26 cases) according to the treatment plan. There
were no significant differences in age, height, body weight, HR,
BSA, Cr and NT-proBNP between the two groups (P>0.05, Table 1).
There were no significant differences in left ventricular structural
parameters (LVEF), VEGF and VEGFR-1 between the control group
and the observation group (P>0.05). (See Tables 1 & 2).
Comparison of Indicators Between the two Groups after
Treatment
After treatment, the cardiac structural parameters (AO, LVIDs,
LA) and lgNT-proBNP in the control group were larger than those
in the observation group, and the systolic function index (LVEF)
was lower than that in the observation group. The difference was
statistically significant (P<0. 05). The VEGF of the control group
was significantly lower than that of the observation group, and the
level of VEGF-1R was higher than that of the observation group, and
the difference was statistically significant (P<0.05) (See Table 3).
Comparison of Indicators in the two Groups after
Treatment
Comparison between the two groups after treatment: Compared
with before treatment, the cardiac structure index and lgNT-proBNP
were significantly decreased in the observation group, and the
systolic function index was significantly increased, the difference
was statistically significant (P<0.05), before treatment. There was
no significant difference in RA between the observation group and
the control group (P>0.05). There were statistical changes between
the control group and other indicators except PWTs, RA, RV, VEGF
and VEGF-1R. Academic significance (P<0.05) (See Table 4).
Correlation Analysis between VEGF and lgNT-proBNP,
VEGF-1 and lgNTproBNP
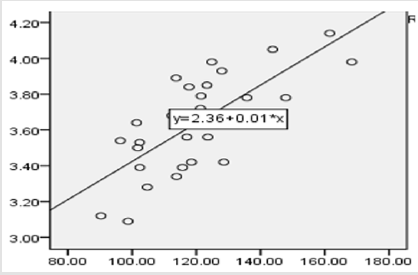
The results showed that VEGF and lgNT-proBNP were positively
correlated after treatment, with a coefficient of 0.534 (P<0.05)
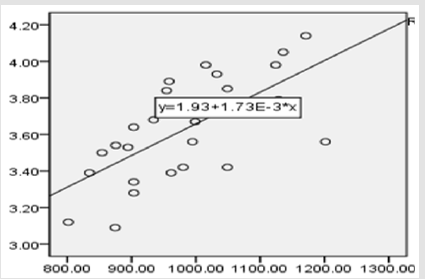
(Figure 1). VEGF-1 and lgNT-proBNP were also positively correlated
after treatment, with a coefficient of 0.459. (P<0.05) (Figure 2).
Heart failure is defined as “a clinical syndrome caused by any
structural or functional heart disease that impairs ventricular
congestion or discharge of blood” [11]. This has been translated
into several validated diagnostic criteria (eg, the Framingham
standard [12] and the European Society of Cardiology standards
[13]). Early studies of heart failure after Myocardial Infarction used
clinical criteria such as the classification criteria of Killip and the
New York Heart Association [14,15]. Although succinct, the Killip
grading retains prognostic value in recent cohort studies (eg, the
GRACE registry): hospitalization mortality in patients with Killip
class I is 3%, and in patients with class III is 20% [16]. In patients
with PCI, a higher Killip grading is an independent predictor of
hospitalization and 6-month mortality [17]. The development of
echocardiography has improved the clinical HF score, which led
to an objective measurement of ejection fraction and ventricular
volume, which is an intrinsic part of HF diagnosis [18].
There are several overlapping mechanisms of HF after
myocardial infarction. Early onset of HF in acute Myocardial
Infarction is due to myocardial shock, myocardial necrosis,
previous HF decompensation or a combination of acute mitral regurgitation due to papillary muscle dysfunction. Heart failure
during hospitalization may also be due to any of the above reasons,
accompanied by fluid or contrast overload, renal insufficiency
or complications such as ventricular septal defect or cardiac
tamponade. Late stages of heart failure reflect the consequences
of simultaneous myocardial cell death and scar formation and
ventricular remodeling. In the 1960s, Killip first used heart
failure after Myocardial Infarction as a prognostic feature of poor
prognosis. Heart failure is associated with extensive Myocardial
Infarction infarction and multivessel disease, and impaired
ventricular function leads to increased mortality [19]. Therefore,
the treatment of heart failure after Myocardial Infarction remains
a major challenge.
Sacubitril/valsartan is the first renin-angiotensinaldosterone
system (RAAS) and enkephalinase double blocker,
and its complementary mechanisms overlap. It is currently the
“breakthrough” drug for the treatment of heart failure. The 2016
European Society of Cardiology Guidelines for the Diagnosis and
Treatment of Acute and Chronic Heart Failure [20] is the first
recommendation to use Sacubitril/valsartan for heart failure.
Enkephalinase [8] is a membrane-bound endopeptidase that
hydrolyzes the atria, brain and C-type natriuretic peptides and other
endogenous vasodilators, such as adrenomedullin and bradykinin,
to clear the above peptides. The main enzyme of the class. Thus,
inhibition of enkephalinase leads to elevated levels of natriuretic
peptides, with several potential benefits such as diuretic effects
and vasodilation [7], dual inhibition of RAAS and enkephalinase
translates into angiotensin II-mediated hypertrophy or fibrosis, as
well as beneficial anti-proliferative and anti-hypertrophic effects.
In general, natriuretic peptides are secreted by excessive blood
volume and increased left ventricular filling pressure, which is a
common feature in patients with heart failure. Therefore, Shakuba
Qusarsartan helps regulate sodium and water balance, blood
volume, arterial blood pressure and sympathetic inhibition. The
PARADIGM HF study demonstrates the striking clinical benefits of
LCZ696, primarily because of a significant reduction in the primary
composite endpoint of cardiovascular death or HF hospitalization,
and a reduced risk of death for any reason. Although the clinical
benefits of shakuba/valsartan have been well documented to
support the expected potential physiological mechanisms, other
effects on tissue remodeling have not been well documented [21].
The mammalian genome encodes five VEGF family members,
VEGF-A (also known as VEGF), placental growth factor (PlGF),
VEGF-B, VEGF-C and VEGF-D, which regulate angiogenesis,
angiogenesis and lymphangiogenesis [22, 23].
In particular, VEGF-A is critical for angiogenesis during early
embryogenesis. Due to the formation of immature blood vessels,
not only VEGF-A homozygous knockout mice but also heterozygous
mice (VEGF-A +/-) showed an embryonic lethal phenotype,
indicating that VEGF-A in embryos must be strictly controlled [24,
25]. Several VEGF-A subtypes were generated by alternative splic ing. Among them, VEGF-A165 has the highest biological activity and
has binding affinity to the co-receptor Neuropilin-1 (Nrp1). Recently,
another spliced form of VEGF-A, VEGFxxxb, has been reported.
VEGFxxxb has a lower affinity for the receptor and competes with
VEGF-A, thereby negatively regulating angiogenesis [26].
In 1990, researchers isolated a gene encoding a novel Tyrosine
Kinase (TK) receptor from human placenta. The TK receptor has
seven immunoglobulin (Ig)-like domains in the extracellular region,
while the TK domain has a 60 amino acid long kinase insert [27].
Based on structural similarity, we named it Fms-like TK-1 (Flt-1). In
1992, Flt-1 was shown to bind to VEGF/vascular permeability factor
and is now known as VEGFR-1 [28]. Unlike VEGFR-2, VEGFR-1 has
a high affinity for its ligand VEGF, and its affinity is about an order
of magnitude higher than that of VEGFR-2 [29]. However, the kinase
activity of VEGFR-1 is low, about one tenth of that of VEGFR-2.
The VEGFR-1 gene produces two major proteins: the full-length
receptor and sFlt-1 [30]. These facts suggest that VEGFR-1 may
have a negative regulatory effect on angiogenesis in some cases.
The study found that VEGFR-1 negatively regulates angiogenesis
during early embryogenesis by capturing VEGF and decreasing the
pro-angiogenic signal of VEGFR-2 [31].
The study found that LCZ696 acts in parallel with the NO-sGCcGMP
pathway by activating the NP-pCG-cGMP signaling system,
which increases diuresis and vasodilation. They also enhance myocardial
relaxation through signaling pathways that are dependent
on cardiac endothelial cells. Increasing cGMP production to reduce
cardiac hypertrophy [32], inhibition of angiotensin receptors
leading to inhibition of the renin-angiotensin-aldosterone system,
which has been shown to be beneficial in heart failure, is consistent
with our findings. Shakuba Qusarsartan improved left ventricular
remodeling. The heart is a muscle pump composed of cardiomyocytes,
Endothelial Cells (EC), fibroblasts, stem cells and inflammatory
cells [33]. Cardiac tissue is a highly organized structure of cells
and extracellular matrices with complex multi-directional communication
between cells. All cells present in the myocardium secrete
autocrine, juxtaposition and paracrine factors that regulate the
function of neighboring cells. Intercellular communication plays a
crucial role in the development of heart and normal heart function
in adult organisms, and plays a crucial role in the pathophysiology
of cardiac remodeling and heart failure development. In particular,
factors secreted by cardiac microvascular EC play a crucial
role in normal cardiac function and cardiac remodeling. During the
pathophysiological development of heart failure, changes in hemodynamic
and mechanical factors, as well as hypoxia, stimulate cardiomyocytes
to release angiogenic growth factors, thereby inducing
parallel growth of the supply vessels.
Vice versa, activated or dysfunctional Endothelial Cells may
also affect the function of other types of cells in the heart. The
study found that the pro-angiogenic effect of β-adrenergic receptor
blockade in rat hearts was reduced by the administration of bait Vascular Endothelial Growth Factor (VEGF) receptor (Ad-Flk)
[34]. The positive nutrient effect of endothelial cell-derived nitric
oxide (NO) leads to earlier relaxation episodes and longer diastolic
phase [35] may also play a role in stimulating cardiac angiogenesis.
Therefore, we found that the VEGF level in the observation group
was significantly higher than that in the control group. Therefore,
it is speculated that LCZ696 activates the NP-pCG-cGMP signaling
system, activates Endothelial Cells to produce VEGF, stimulates
angiogenesis and improves cardiomyocyte hypertrophy, and can
pass endothelial cells. The paracrine function and intercellular
signal series delay the fibrosis of cardiomyocytes; while the
improvement of myocardial blood flow and left ventricular function
directly stimulates the endothelial erbB receptor through autocrine,
indirectly by increasing the expression of VEGF and angiopoietin-1
[36].
Cardiac Endothelial Cells not only respond to hemodynamic
forces and paracrine signals of adjacent cells, but also actively
participate in the cardiac remodeling process by stimulating the
growth and contraction of cardiomyocytes or the production of extracellular
matrix proteins in myoblasts. Furthermore, in response
to appropriate signals, they may alter their phenotype and differentiate
into extracellular matrix producing cells. Since cardiac angiogenesis
plays a central role in the transition from adaptive cardiac
hypertrophy to heart failure, Endothelial Cells and signaling
mechanisms involved in the regulation or regulation of cardiac angiogenesis
represent the potential to improve cardiac remodeling
and prevent stress overload. A therapeutic target for arrhythmias.
Han Xia designed this research. Wu Menghai collected samples
and clinical data. Qi Huiqin and Liu Wenting carried out experiments.
Wu Menghai analyzed the data, Han Xia wrote the paper.
The authors would like to thank Karin Fava for proofreading
the article.
The authors declare that the research was conducted in the
absence of any commercial or financial relationships that could be
construed as a potential conflict of interest.
- Nabel EG, Braunwald E (2012) A tale of coronary artery disease and myocardial infarction. N Engl J Med366(1):54-63.
- Smilowitz NR, Feit F (2016) The History of Primary Angioplasty and Stenting for Acute Myocardial Infarction. Curr Cardiol Rep18(1):5.
- Keeley EC, Boura JA, Grines CL (2003) Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet361(9351):13-20.
- Dehmer GJ, Weaver D, Roe MT, MilfordBeland S, Fitzgerald S, et al. (2012) A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: a report from the CathPCI Registry of the National Cardiovascular Data Registry, 2010 through June 2011. J Am Coll Cardiol60(20):2017-2031.
- Jernberg T, Johanson P, Held C, Svennblad B, Lindbäck J, et al. (2011) Association between adoption of evidence-based treatment and survival for patients with STelevation myocardial infarction. JAMA305(16):1677-1684.
- Puymirat E, Simon T, Steg PG, Schiele F, Guéret P, et al. (2012) Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA308(10):998-1006.
- Gu J, Noe A, Chandra P, AlFayoumi S, LiguerosSaylan M, et al. (2010) Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol50(4):401-414.
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, et al. (2013) Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail15(9): 1062-1073.
- Pitzalis MV, Iacoviello M, Romito R, Guida P, De Tommasi E, et al. (2005) Ventricular asynchrony predicts a better outcome in patients with chronic heart failure receiving cardiac resynchronization therapy. J AmColl Cardiol45(1):65-69.
- Cintron G, Johnson G, Prancis G, Cobb F, Cohn JN (1993) Prognostic significance of serial changes in left ventricular ejection fraction in patients with congestive heart failure. The VHeFT VA Cooperative Studies Group Circulation87(suppl):VI17-VI23.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, et al. (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol62(16): e147-e239.
- McKee PA, Castelli WP, McNamara PM, Kannel WB (1971) The natural history of congestive heart failure: the Framingham study. N Engl J Med285(26):1441-1446.
- Swedberg K, Cleland J, Dargie H, Drexler H, Follath F,et al. (2005) Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J26(11):1115-1140.
- Killip T, Kimball JT (1967) Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol20(4):457-464.
- Spencer FA, Meyer TE, Goldberg RJ, Yarzebski J, Hatton M, et al. (1999) Twenty year trends (1975-1995) in the incidence, in-hospital and long-term death rates associated with heart failure complicating acute myocardial infarction: a community-wide perspective. J Am Coll Cardiol34(5):1378-1387.
- Steg PG, Dabbous OH, Feldman LJ, CohenSolal A, Aumont MC, et al. (2004) Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE) Circulation109(4):494-499.
- DeGeare VS, Boura JA, Grines LL, ONeill WW, Grines CL (2001) Predictive value of the Killip classification in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol87(9):1035-1038.
- Nicod P, Gilpin E, Dittrich H, Chappuis F, Ahnve S, et al. (1988) Influence on prognosis and morbidity of left ventricular ejection fraction with and without signs of left ventricular failure after acute myocardial infarction. Am J Cardiol61(15):1165-1171.
- (1983) Risk stratification and survival after myocardial infarction. N Engl J Med309(6):331-336.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, et al. (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology(ESC).Developed with the special contribution of the Heart Failure Association(HFA)of the ESC.Eur J Heart Fail 37(27): 2129-2200.
- Fonarow GC, Hernandez AF, Solomon SD, Yancy CW (2016) Potential mortality reduction with optimal implementation of angiotensin receptor neprilysin inhibitor therapy in heart failure. JAMA Cardiol1(6):714-717.
- Ferrara N, Kerbel RS (2005) Angiogenesis as a therapeutic target. Nature438(7070):967-974.
- Shibuya M (2011) Involvement of Flt-1 (VEGFR-1) in cancer and preeclampsia. Proc Jpn AcadSer B Phys BiolSci87(4):167-178.
- Ferrara N, CarverMoore K, Chen H, Dowd M, Lu L, et al. (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature380(6573):439-442.
- Carmellet P, Ferreira V, Breier G, Pollefeyt S, Kleckens L, et al. (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature380(6573):435-439.
- PritchardJones RO, Dunn DB, Qiu Y, Varey AH, Orlando A, et al. (2007) Expression of VEGF(xxx)b, the inhibitory isoforms of VEGF, in malignant melanoma. Br J Cancer97(2):223-230.
- Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, et al. (1990) Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene5(4):519-524.
- De Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, et al. (1992) The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science255(5047):989-991.
- Sawano A, Takahashi T, Yamaguchi S, Aonuma T, Shibuya M (1996) Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for Placenta Growth Factor (PlGF), which is related to Vascular Endothelial Growth Factor (VEGF) Cell Growth Diff7(2): 213-221.
- Kendall RL, Thomas KA (1993) Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci90(22):10705-10709.
- Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M (1998) Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci95(16):9349-9354.
- Potter LR, AbbeyHosch S, Dickey DM (2006) Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev27(1):47-72.
- Kamo T, Akazawa H, Komuro I (2015) Cardiac nonmyocytes in the hub of cardiac hypertrophy.Circ Res117(1): 89-98.
- Rengo G, Cannavo A, Liccardo D, Zincarelli C, De Lucia C,et al. (2013) Vascular endothelial growth factor blockade prevents the beneficial effects of beta-blocker therapy on cardiac function, angiogenesis, and remodeling in heart failure. Circ Heart Fail6(6):1259-1267.
- Gyurko R, Kuhlencordt P, Fishman MC, Huang PL (2000) Modulation of mouse cardiac function in vivo by eNOS and ANP. Am J Physiol Heart Circ Physiol278(3):H971-981.
- Gui C, Zeng ZY, Chen Q, Luo YW, Li L, et al. (2018) Neuregulin-1 promotes myocardial angiogenesis in the rat model of diabetic cardiomyopathy. Cell Physiol Biochem46(6):2325-2334.

 Research Article
Research Article