Abstract
Pyruvate present in biological fluids represents a useful biomarker for inferring cellular redox state, mitochondrial aerobic capacity and response to exercise in both a native and supplemented state. Commonly pyruvate is assessed intracellularly via muscle biopsy or in blood/plasma. We review a non-invasive means of monitoring perturbations to intracellular and blood pyruvate levels indirectly by monitoring alterations of pyruvate in perspiration during the course of physical exertion (i.e., exercise or strenuous activity) over an assessed time-period in both a native and supplemented state. Pyruvate as a biomarker can allow the assessment of exercise performance and recovery, permitting the determination of the effectiveness of metabolic intervention (supplementation) in a non-invasive manner in bodily sweat. Pyruvate in sweat may also be used to infer disease states or monitor supplementation levels in other regimes other than exercise.
Introduction
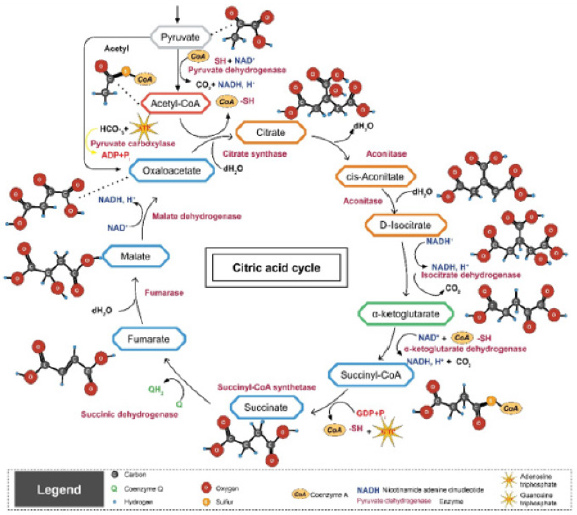
Pyruvate, representing the terminal metabolite in glycolysis and the key molecule reacted upon by the pyruvate dehydrogenase complex to initiate the first step of the Kreb cycle (Figure 1), linking anaerobic and aerobic metabolic energy generating pathways, is in relative equilibrium with lactate. Cellular lactate is produced when the aerobic capacity of the cell is challenged with increased metabolic demand by cellular work, as reflected by the exercising state or exertion, and correlates with increasing cellular acidosis and diminished aerobic capacity. We review the potential that pyruvate levels in sweat collected and monitored over an exercise period reflect those present in blood and can be modulated by metabolic intervention using a supplement aimed at improving athletic performance. Pyruvate, present in sweat, represents a suitable biomarker to monitor changing intracellular and extracellular pyruvate levels which influence metabolic performance.
Figure 1: Kreb Cycle illustrating the entry point of pyruvate through the pyruvate dehydrogenase complex and subsequent intra-mitochondrial reactions.
Lactic Acidosis Arising from Increased Metabolic Demand
Cellular metabolic acidosis strongly correlates with rising lactate levels [1,2] and reflects the cellular metabolic threshold [3] making lactate a useful indirect biomarker [40] for cellular and mitochondrial performance during periods of cellular stress or alterations to available oxygen tension. Hence, acidosis reflects increased tissue metabolic demand as exemplified by increased muscular contraction, perturbations to the cellular redox state (NAD+: NADH ratio) and ATP hydrolysis during intensive exercise [2]. Similar observations may arise from inborn error of metabolism [4,5], or medical conditions resulting in tissue hypoxia, such as sepsis [6].
It is well established that blood lactate levels rise in response to increased metabolic demand and correlate to increasing cellular acidosis as a means of buffering proton production by increased ATP hydrolysis. In this regard, lactate levels in blood rapidly rise and subsequently decrease as this metabolite is taken up by the liver to replenish glucose by means of glyconeogeneis as an anaplerotic metabolic pathway. Similarly, pyruvate levels in blood and tissue [7] have been shown to increase during the course of exercise, thereby acting as a suitable biomarker to infer cellular metabolic demand.
Metabolites in Sweat
A variety of polar metabolites can be collected and analyzed from the human sweat metabolome including amino acids and various organic acids [8,9]. The human sweat metabolome comprises upwards of 2700 unique compounds [10], but present in relatively high abundance are lactate, pyruvate, betaine, ammonia and urea among others [11,12]. Although these metabolites have been shown to be present in human, perturbations to such metabolites over a time series and modulation by supplementation to these metabolites as a result of physical exertion in both genders have not been demonstrated, particularly for pyruvate which is present in relatively reduced levels compared to lactate.
Exercise and Blood Lactate Biomarker Assessment
Intense exercise dramatically elevates blood lactate levels [13- 22] with levels often increasing across a ten-fold range during bouts of exercise [23], collectively demonstrating that blood lactate levels reflect muscle lactate accumulation. Commonly venous blood [4] or muscle biopsy [24] are used to measure changes to lactate levels, but these metabolite sources are invasive and not conducive to repeated sampling as would be necessary when monitoring changes to exercise induced lactate levels in athletes.
Lactate Biomarker Assessment in Sweat
A readily obtainable bodily fluid, other than urine [16], whose lactate levels may reflect venous concentrations is exercise induced bodily sweat [17]. Although the correlation between blood lactate levels and sweat is debatable [15,25], this sampling source is noninvasive, very conducive to repeat sampling, and minimally perturbs the athlete during performance. Also, variations in sweat lactate levels during exercise can be correlated to sodium abundance [17] to equalize variability in sweat volume, evaporation and concentration differences among individuals. Several studies have shown that lactate levels in sweat correlate with those in venous blood [17,26-28]; and baseline lactate concentrations across the skin surface do not vary [21]. More importantly, skin surface lactate levels correlate with exercise intensity [17] and elevated heartbeat [29]. This correlation has been demonstrated with various sampling modalities and most recently with an epidermal amperometric skin biosensor which exhibited a significant and continual increase in sweat lactate levels during exercise [27]. Finally, a comprehensive sweat metabolome analysis detailed that numerous metabolites, in addition to lactate, are modulated in response to exercise [10], further supporting the use of this bodily fluid for biomarker assessment. Such measurements permit the non-invasive assessment of glycolytic rate [30] and anaerobic work capacity during exercise [31] and can thus be used to infer mitochondrial performance in trained and untrained individuals.
Pyruvate Levels in Blood and Urine During Exercise
Pyruvate levels in blood have been shown to be modulated by exercise, generally increasing during exertion and decreasing postrecovery and can be altered by providing test subjects lactate as a supplement prior to exercise. Modulation of pyruvate in other body fluids, particularly sweat, has not been addressed or considered as reflecting intracellular or blood metabolite levels. Pyruvate ingestion influences blood lactate parameters during and post exercise [16,20]. Johnson and Edwards [16] further demonstrated that urine exhibited a similar profile of lactate and pyruvate increasing during exercise and remained elevated 90 minutes post recovery [16], although sweat or other bodily fluid levels were not considered in this study.
Pyruvate Levels in Sweat During Exercise
Kondoh et al., [19] measured pyruvate levels in sweat and reported an increase in pyruvate levels after the cessation of exercise (recovery period) compared to sauna induced sweat, but the course of modulation and a profile of increasing concentration of pyruvate in sweat was not demonstrated or commented upon. Rather this publication emphasized D-lactate and L-lactate changes in sweat and did not monitor levels over the course of exercise, nor did they consider the influence of supplementation or gender on pyruvate levels. The implications of modulated pyruvate levels at baseline and before and after exertion, were not addressed or fully discussed, rather the investigators emphasized the concentrations of D-lactate as the emphasis of the report. Biagi et al. [23] demonstrated the capability of measuring pyruvate levels in sweat, but reported their data as the lactate-to-pyruvate ratio to show modulation of this ratio during the course of exercise rather than absolute pyruvate concentrations, placing a greater emphasis on method development rather than pyruvate metabolite as its own metabolite as was done for changing lactate levels. The afore mentioned lactate-to-pyruvate ratio modulation may be the result of changing lactate levels, changing pyruvate levels or both metabolites changing simultaneously. A thorough investigation of specific alterations to pyruvate levels in sweat during the course of exercise, either in the native or supplemented state, has not been adequately demonstrated.
Other Conditions Affecting Pyruvate Levels
In plasma of exercised heart failure patients, pyruvate exhibited significant increases post exercise in both patients and controls, and strongly correlated with peak VO2 and circulatory power, which was presented as reflecting its intramuscular concentration [32] and may be significantly impacted during anemic states [33]. Blood levels of pyruvate in patients with mitochondrial myopathy with progressive external ophthalmoplegia may also exhibit alterations to blood pyruvate levels allowing a prognostic application of this metabolite in conjunction with lactate [34]. Interestingly, Kleeburg et al. [35] presented blood pyruvic acid levels were altered essentially within the normal range in blood from renal disease patients in a uremic coma.
Summary and Conclusion
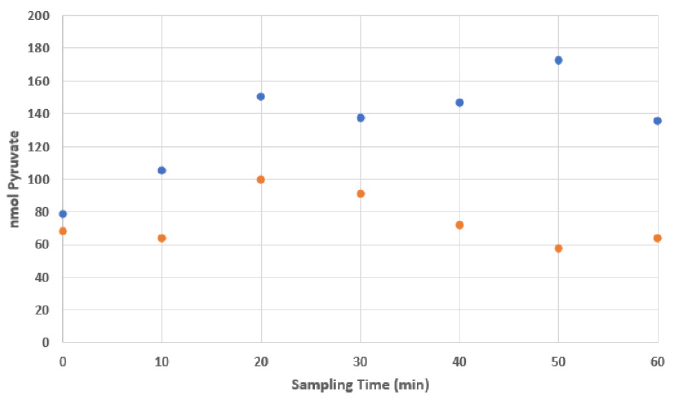
Pyruvate levels in sweat are modulated during the course of exercise and if repeatedly sampled over the course of an exercise session, show concentration changes. For example, in many male athletes an initial increase in pyruvate amounts is followed by a general decrease at the cessation of the assessment period. The absolute levels in subjects can be influenced by supplementation, thereby inferring that supplementation enables metabolic interventions and pyruvate levels in sweat reflect intracellular and/or blood pyruvate and can act as a suitable biomarker to noninvasively monitor a subject’s metabolic profile. Concurrently, the reduced levels of pyruvate in sweat post-exertion reflect the putative flux of metabolites through the Kreb cycle and influence mitochondrial performance and recovery. Figure 2. Example of pyruvate accumulation in sweat over exercise in a male athlete supplemented with a Health Canada approved natural supplement (NPN80070757). Pyruvate levels were determined chemically according to the procedure of Sharma and Lee [36], whereby the assay was optimized for sweat samples and volumes. Sweat was collected on a standard Band Aid TM brand bandage applied to the upper back region of the participant following a thorough cleaning of the site with a 70% isopropanol swab. Post exercise each bandage was retrieved and stored frozen at -80⁰C until analyzed. Sweat was retrieved by aseptically removing the absorbent pad from the adhesive backing and placing the pad in a sterile 3 mL syringe barrel to which was attached a 0.22 μm low retention filter. The pad was rehydrated in situ with 1 mL of milliQ water and the soluble metabolites pressure extruded by forcefully applying the plunger. Extraction from a comparable unused Band Aid TM acted as a control [36-39].
Figure 2: Example of pyruvate accumulation in sweat over exercise in a male athlete supplemented with a Health Canada approved natural supplement (NPN80070757).
Acknowledgement
We wish to thank the support and excellent technical support from Mr. Bruce Griffin and the assistance form Ms. Shuk YeeNgan and Ms. Kamilia Talipova. We also greatly appreciate the continued support from Ms. Paola Battiston, Chair, Seneca, School of Biological Sciences and Applied Chemistry and the staff comprising the Division of Applied Research, Innovation and Entrepreneurship.
Conflict of Interest
No conflict of interest.
References
- Robinson BH, Mc Kay N, Goodyer P, Lancaster G (1985) Defective intramitochondrial NADH oxidation in skin fibroblasts from an infant with fatal neonatal lacticacidemia. Am J Hum Genet 37(5): 938-946.
- Robinson BH (2006) Biochemistry of exercise-induced metabolic acidosis. Molecular Genetics and Metabolism 89: 3-13.
- Neill WA, Jensen PE, Rich, GB, Wemsckl JD (1969) Effect of Decreased 02 Supply to Tissue on the Lactate: Pyruvate Ratio in Blood. The Journal of Clinical Investigation 48.
- Debray FG, Mitchell GA, Allard P, Robinson BH, Hanley JA, et al. (2007) Diagnostic accuracy of blood lactate-to-pyruvate molar ratio in the differential diagnosis of congenital lactic acidosis. Clin Chem 53(5): 916-921.
- Sharma K, Lee YR (2016) Effect of different storage temperature on chemical composition of onion (Allium cepa L.) and its enzymes. J Food Sci Technol 53(3): 1620-1632.
- Van Meerhaeghe A, Velkeniers B (2005) Lactate production and exercise-induced metabolic acidosis: guilty or not guilty? Eur Respir J 26(4): 744.
- Dohm GL, Patel VK, Kasperek GJ (1986) Regulation of muscle pyruvate metabolism during exercise. Biochem Med Metab Biol 35(3): 260-266.
- Calderón Santiago M, Priego Capote F, Jurado Gámez B, Luque de Castro MD (2014) Optimization study for metabolomics analysis of human sweat by liquid chromatography-tandem mass spectrometry in high resolution mode. J Chromatogr A 1333: 70-78.
- Douglas P Lee, Adam D Kennedy, Eric K ONeal, Phillip A Bishop, Mark D Haub, et al. (2011) Global untargeted metabolic profiling of human sweat from exercising men and women. International Society of Sports Nutrition: 8th Annual ISSN Conference and Expo Las Vegas, NV, USA, p. 24-25.
- Jia W, Bandodkar AJ, Valdés Ramírez G, Windmiller JR, Yang Z, et al. (2013) Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal Chem 85(14): 6553-6560.
- Melenovsky V, Kotrc M, Polak J, Pelikanova T, Bendlova B, et al. (2012) Availability of energetic substrates and exercise performance in heart failure with or without diabetes. Eur J Heart Fail 14(7): 754-763.
- Ohkuwa T, Tsukamoto K, Yamai K, Itoh H, Yamazaki Y, et al. (2009) The Relationship between Exercise Intensity and Lactate Concentration on the Skin Surface. Int J Biomed Sci 5(1): 23-27.
- Almeida JA, Petriz Bde A, Da Costa Gomes CP, Pereira RW, Franco OL (2012) Assessment of maximal lactate steady state during treadmill exercise in SHR. BMC Res Notes 5: 661.
- Goodwin ML, Harris JE, Hernández A, Gladden LB (2007) Blood lactate measurements and analysis during exercise: a guide for clinicians. J Diabetes Sci Technol 1(4): 558-569.
- Green JM, Bishop PA, Muir IH, Mc Lester JR, Heath HE (2000) Effects of high and low blood lactate concentrations on sweat lactate response. Int J Sports Med 21(8): 556-560.
- KLEEBERG J, GITELSON S (1956) The blood pyruvic acid level in renal diseases and in uraemic coma. J Clin Pathol 9(2): 148-152.
- Medbø JI (1993) Glycogen breakdown and lactate accumulation during high intensity cycling. Acta Physiol Scand 149: 85-89.
- Meyer F, Laitano O, Bar-Or O, Mc Dougall D, Heigenhauser GJ (2007) Effect of age and gender on sweat lactate and ammonia concentrations during exercise in the heat. Braz J Med Biol Res 40(1): 135-143. Erratum in: Braz J Med Biol Res 40(6): 885
- Patterson MJ, Galloway SD, Nimmo MA (2000) Variations in regional sweat composition in normal human males. Exp Physiol 85(6):869-875.
- Pilardeau PA, Chalumeau MT, Harichaux P, Vasseur P, Vaysse J, et al. (1988) Effect of physical training on exercise induced sweating in men. J Sports Med Phys Fitness 28(2): 176-180.
- Robergs RA (2001) Sport Science 5(2).
- Tesch PA, Daniels WL, Sharp DS (1982) Lactate accumulation in muscle and blood during submaximal exercise. Acta Physiol Scand114(3): 441-446.
- Biagi S, Ghimenti S, Onor M, Bramanti E (2012) Simultaneous determination of lactate and pyruvate in human sweat using reversed-phase high-performance liquid chromatography: a noninvasive approach. Biomed Chromatogr 26(11): 1408-1415.
- Yoshikawa H, Sato T, Fukuyama T (1949) The Level of Pyruvic and Lactic Acids Following Muscular Activity and Ingestion of Lactate. The Japanese Medical Journal 2(1): 32-37.
- Derbyshire PJ, Barr H, Davis F, Higson SP (2012) Lactate in human sweat: a critical review of research to the present day. J Physiol Sci 62(6): 429-440.
- Buono MJ, Lee NV, Miller PW (2010) The relationship between exercise intensity and the sweat lactate excretion rate. J Physiol Sci 60(2): 103-107.
- Robergs RA, Ghiasvand F, Parker D (2004) Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol 287(3): R502-16.
- Smekal G, Von Duvillard SP, Pokan R, Hofmann P, Braun WA, et al. (2012). Blood lactate concentration at the maximal lactate steady state is not dependent on endurance capacity in healthy recreationally trained individuals. Eur J Appl Physiol 112(8): 3079-3086.
- Johnson RE, Edwards HT (1937) Lactate and Pyruvate in blood and urine after exercise. Journal of Biological Chemistry 118: 427-432.
- Menzies P, Menzies C, Mc Intyre L, Paterson P, Wilson J, et al. (2010) Blood lactate clearance during active recovery after an intense running bout depends on the intensity of the active recovery. Journal of Sports Sciences28 (9): 975-982.
- Fujitsuka N, Yamamoto T, Ohkuwa T, Saito M, Miyamura M (1982) Peak blood lactate after short periods of maximal treadmill running. Eur J Appl Physiol 48: 289-296.
- Myers J, Ashley E (1997) Dangerous curves. A perspective on exercise, lactate, and the anaerobic threshold. Chest 111(3): 787-795.
- Olek RA, Kujach S, Wnuk D, Laskowski R (2014) Single sodium pyruvate ingestion modifies blood acid-base status and post-exercise lactate concentration in humans. Nutrients 6(5):1981-1992.
- Dengler R, Wohlfarth K, Zierz S, Jobges M, Schubert M (1996) Muscle fatigue, lactate, and pyruvate in mitochondrial myopathy with progressive external ophthalmoplegia. Muscle Nerve 19(4): 456-462.
- Lee DP, Kennedy AD, O’Neal EK, Bishop PA, Haub MD, et al. (2011) Global untargeted metabolic profiling of human sweat from exercising men and women. J Int Soc Sports Nutr 8(Suppl 1): P9.
- Suetrong B, Walley KR (2016) Lactic Acidosis in Sepsis: It's Not All Anaerobic: Implications for Diagnosis and Management. Chest 149(1): 252-261
- Hooton K, Han W, Li L (2016) Comprehensive and Quantitative Profiling of the Human Sweat Submetabolome Using High-Performance Chemical Isotope Labeling LC-MS. Anal Chem 88(14): 7378-7386.
- Kondoh Y, Kawase M, Ohmori S (1992) D-lactate concentrations in blood, urine and sweat before and after exercise. Eur J Appl Physiol Occup Physiol 65(1): 88-93.
- Sakharov DA, Shkurnikov MU, Vagin MY, Yashina EI, Karyakin AA, et al. (2010) Relationship between lactate concentrations in active muscle sweat and whole blood. Bull Exp Biol Med 150(1): 83-85.

 Review Article
Review Article