Abstract
Background: Many studies suggest that pancreatic cancer may be associated with hepatitis B virus (HBV) infection. However, the results are still controversial. We carried out a meta-analysis to assess the association between HBV infection and risk of pancreatic cancer.
Methods: All relevant studies that have reported an association between HBV infection and risk of pancreatic cancer before April 2019 were identified by a systematic search of PubMed and Cochrane Library databases. The Newcastle-Ottawa quality assessment Scale (NOS) was used to assess the quality of included studies. A fixed-effect model or random-effect model was used to conduct a meta-analytic estimate.
Results: Nine case-control studies and three cohort studies were included. We found that hepatitis B surface antigen (HBsAg) positive patients was significantly increased risk of pancreatic cancer with OR of 1.19 (95% CI: 1.07-1.31), especially in the Asian population (OR=1.18, 95% CI: 1.06-1.31). However, past exposure to HBV with natural immunity [Anti-HBc positive/Anti-hepatitis B surface antigen (Anti-HBs) positive] had no association with the development of pancreatic cancer (OR=1.31, 95%CI: 0.79- 2.20), nor did past exposure to HBV without natural immunity [Anti-HBc positive/Anti- HBs negative], with OR 1.67 (95%CI: 0.95-2.91). There was significant heterogeneity between these studies. Nevertheless, no evidence of publication bias between HBV infection and pancreatic cancer was found.
Conclusion: HBV infection have an increased risk of pancreatic cancer. Our findings require further confirmation by large numbers of population-based multicenter prospective studies.
Keywords: Hepatitis B virus; Pancreatic Cancer; Meta-Analysis
Introduction
Pancreatic cancer is a solid malignant tumor with a poor prognosis, almost all patients died within 1 year after diagnosis, with an overall 5-year survival rate less than 5% [1]. Although pancreatic cancer has been studied at the molecular level, its etiology is still unknown. At present, only a few risk factors related to pancreatic cancer have been identified, such as smoking, alcoholism, diabetes mellitus, obesity and chronic pancreatitis [2-4]. Therefore, it is important to further explore the pathogenic factors. In recent years, many observational studies have suggested that the incidence of pancreatic cancer may be related to HBV infection [5,6- 10]. However still other studies hold an opposite opinion that there was no association between HBV infection and risk of pancreatic cancer [11-18]. Therefore, we conducted a comprehensive analysis and evaluation in order to confirm the association between HBV infection and risk of pancreatic cancer.
Materials and Methods
Search Strategy
A systematic literature retrieval was carried out on case-control or cohort studies from PubMed and cochrane library databases, without language or time limit. The following Keywords or Medical Subject Heading (MeSH) terms were used: hepatitis B OR hepatitis B virus OR HBV OR hepatitis B surface antigen OR HBsAg OR hepatitis B virus DNA, pancreatic cancer OR pancreatic neoplasm. References cited in the selected studies were also manually screened.
Selection Criteria
Studies were included in this meta-analysis if they met the following criteria:
1) Study design was case-control or cohort studies.
2) Research objectives were to assess the association between HBV infection and risk of pancreatic cancer.
3) Diagnosis of pancreatic cancer was based on pathological and/or clinical imaging examinations.
4) There were serological tests for HBV infection.
5) Research results should have sufficient information to calculate the OR or RR and 95%CI.
Data Extraction
Data were extracted from each study by two investigators (Xujian Chen and Jing Wang) independently, including the first author’s last name, publication year, country or region, study design, test items of HBV, diagnostic criteria of pancreatic cancer, sample size, OR or RR of pancreatic cancer and corresponding 95%CI, and covariates adjusted for in the analysis. Any discrepancies were resolved by consensus.
Quality Assessment
The NOS [19] was used to assess the quality of the included studies, which consists of 8 questions with 9 possible points. The assessment score ranged from 0 to 9. Studies with a total score of 6 or higher indicated high quality study.
Statistical Analysis
Statistical analyses were performed by using STATA version 15.1 (StataCorp LLC, College Station, TX, USA). The odd ratios were used to analyze the statistics and corresponding 95%CIs were record. Between-study heterogeneity was estimated by chisquare test. If the heterogeneity test results were not statistically significant (P > 0.1, I2 < 50%), the fixed-effect model was used for meta-analysis. On the contrary, the random-effect model was used. The potential publication bias was assessed using both Begg’s funnel plot and Egger’s test.
Results
Eligible Studies
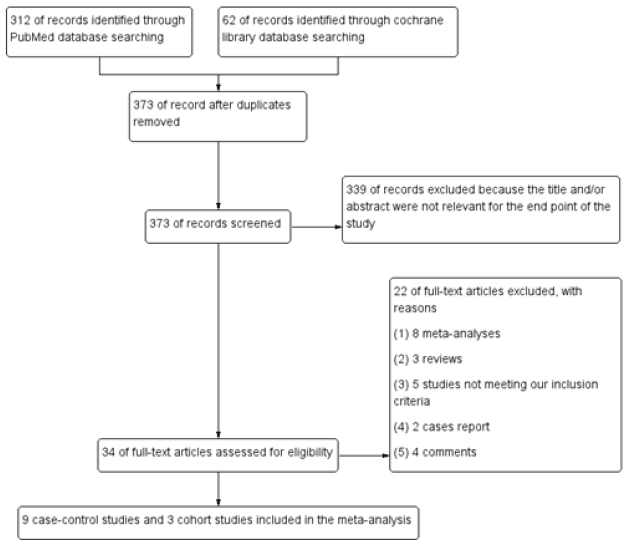
The results of literature retrieval is show in Figure 1. A total of 12 eligible studies were included. 361 of other articles were excluded because of the following reasons:
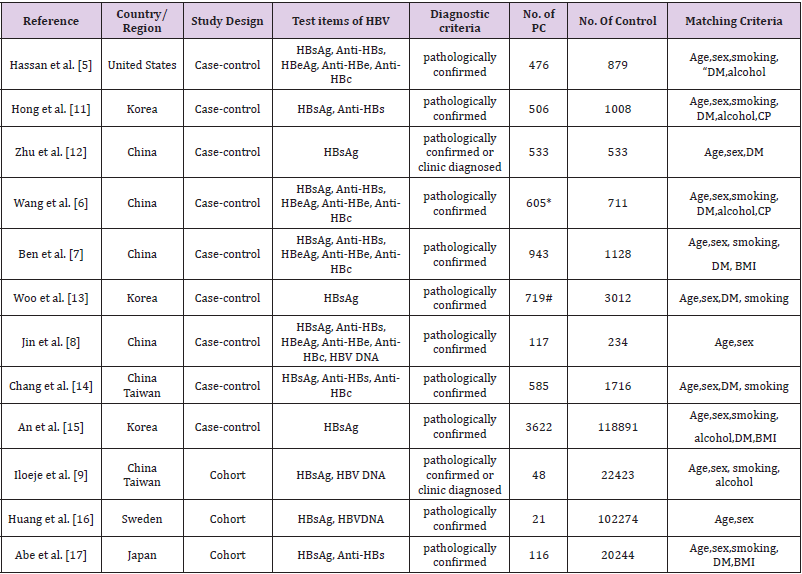
The characteristics of included studies are pooled in Table 1. Nine studies were case-control, and three were cohort studies published between 2008 and 2018. Six studies were conducted in China, three in Korea, one in Japan, one in Sweden and one in the United States. These studies included a total of 281 344 investigated patients, including 8 291 patients with pancreatic cancer. In most of studies [5-8,11,13-17], pancreatic cancer was confirmed by pathology. In other studies [9,12], pancreatic cancer was confirmed by pathology or clinic.
Table 1: Characteristics of all studies included in the analysis.
Note: HBV: Hepatitis B Virus; HBsAg: Hepatitis B Surface Antigen; Anti-HBs: Anti-Hepatitis B Surface Antigen; HBeAg: Hepatitis B e Antigen; Anti-HBe: Anti-Hepatitis B e Antigen; Anti-HBc: Anti-Hepatitis B Core Antigen. PC: Pancreatic Cancer; DM: Diabetes Mellitus; BMI: Body Mass Index; CP: Chronic Pancreatitis; *40 Cases Lacked HBV serologic testing data were not added for analysis; #34 cases of data on HBsAg were missing.
Quality Assessment of Included Studies
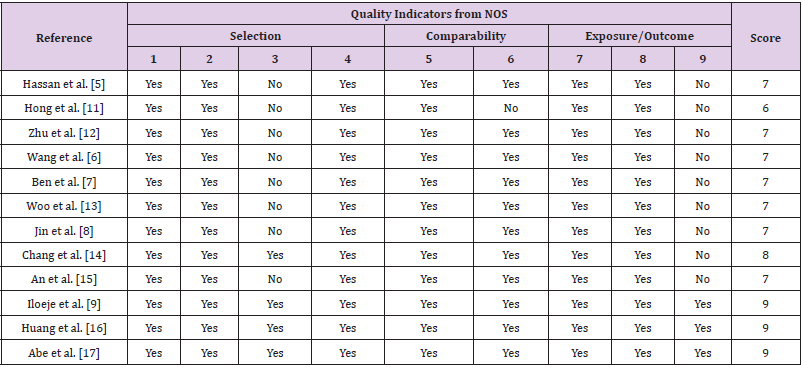
The results of quality assessment of each study are tabulate in Table 2. All included studies scored 6 or higher.
Table 2: Quality assessment of included studies. NOS. For case-control studies:
(1) the case definition adequate, with independent validation;
(2) cases are consecutive or representative;
(3) community controls ;
(4) controls have no history of pancreatic cancer;
(5) study controls are comparable for age and sex;
(6) study controls for any additional factor(s);
(7) cases and controls have the same method of ascertainment;
(8) was follow-up long enough for outcomes to occur; and
(9) cases and controls have complete follow-up.
For cohort studies:
(1) indicates the exposed cohort study representative of the population;
(2) the non-exposed cohort drawn from the same population;
(3) the exposure ascertainment are from secure record or structured interview;
(4) the pancreatic cancer was not present at start of study;
(5) cohorts are comparable for age and sex;
(6) cohorts are comparable for any additional factor(s);
(7) assessment of pancreatic cancer is from secure record;
(8) follow-up long enough for pancreatic cancer to occur; and
(9) complete follow-up.
Data Synthesis
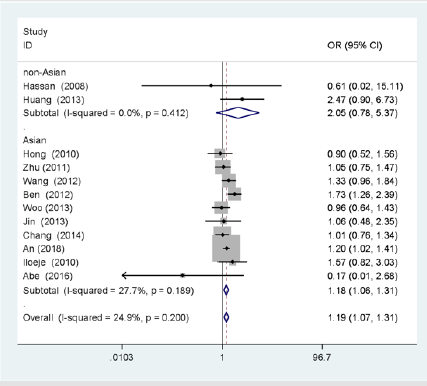
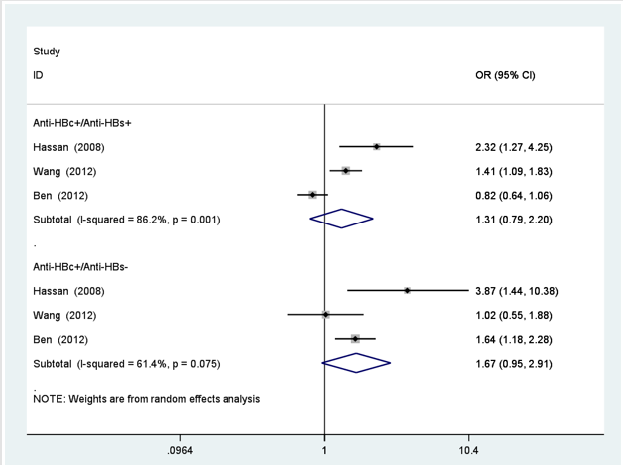
In Figure 2, the overall OR was estimated for HBsAg positive patient’s vs negative people from all the included studies. The pooled OR of pancreatic cancer from all the studies was 1.19 (95%CI: 1.07-1.31). There was no significant heterogeneity among studies (I2=24.9%, P=0.200). Figure 2 also showed the ORs from the studies conducted in Asian and two studies conducted in non-Asian. The pooled OR of Asian was 1.18 (95%CI: 1.06-1.31) for HBsAg positive patients, and OR of non-Asian was 2.05 (95%CI:0.78-5.37). There was no significant heterogeneity between the ten studies in Asian (I2=27.7%, P=0.189) and two studies in non-Asian (I2=0%, P=0.421). In Figure 3, the risk of pancreatic cancer was not observed for anti-HBc positive/anti-HBs positive patients who were previously exposed to HBV with natural immunity, with OR of 1.31 (95%CI: 0.79-2.20) (I2=86.2%, P=0.001), nor did past exposure to HBV without natural immunity (anti-HBc positive/anti-HBs negative), with OR of 1.67 (95%CI: 0.95-2.91) (I2=61.4%, P=0.075).
Figure 2: Forest plot showing overall and subgroup analysis on the association between HBsAg positive state and risk of pancreatic cancer according to region.
Figure 3: Forest plots of risk of pancreatic cancer. associated with past exposure to HBV with or without natural immunity.
Stratified Analysis
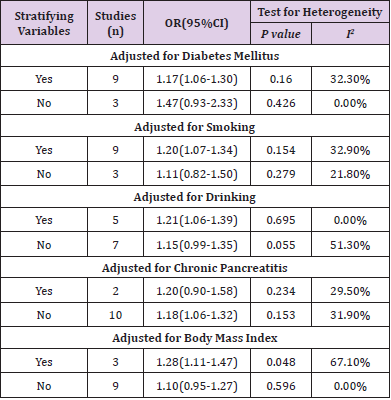
We conducted a stratified analysis to assess the impact of confounding factors on HBsAg positive status, as shown in Table 3. When we limited the meta-analysis to these studies that adjusted for diabetes mellitus, the association between inactive and chronic HBV infection and risk of pancreatic cancer was positive, with a pooled OR of 1.17 (95%CI: 1.06-1.30) (I2=32.3%, P=0.160). There was no positive association between inactive and chronic HBV infection and risk of pancreatic cancer in the studies that did not adjust for diabetes mellitus, with a pooled OR of 1.47 (95%CI: 0.93-2.33) (I2=0.0%, P=0.426). Likewise, the association between inactive and chronic HBV infection and risk of pancreatic cancer was also positive between these studies that were adjusted for smoking (OR=1.20, 95%CI: 1.07-1.34, I2=32.9%, P=0.154), drinking (OR=1.21, 95%CI: 1.06-1.39, I2=0.0%, P=0.695), body mass index (OR=1.28, 95%CI: 1.11-1.47, I2=67.1%, P=0.048) and those that were not adjusted for smoking (OR=1.11, 95%CI: 0.82- 1.50, I2=21.8%, P=0.279), drinking (OR=1.15, 95%CI: 0.99-1.35, I2=51.3%, P=0.055), body mass index (OR=1.10, 95%CI: 0.95-1.27, I2=0.0%, P=0.596). However, when we limited the meta-analysis to these studies that adjusted for chronic pancreatitis, there was no positive association between inactive and chronic HBV infection and risk of pancreatic cancer, with a pooled OR of 1.20 (95%CI: 0.90-1.58) (I2=29.5%, P=0.234).
Publication Bias
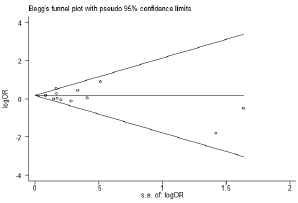
According to the results of Begg’s and Egger’s test, there was no publication bias was found Figure 4, (Begg’s test, P=0.837, Egger’s test, P=0.686).
Figure 4: Forest plots of risk of pancreatic cancer. associated with past exposure to HBV with or without natural immunity.
1) 339 articles were not relevant for the end point of the study.
2) 8 articles were meta-analyses.
3) 3 were reviews.
4) 5 studies not meeting our inclusion criteria.
5) 2 were case report.
6) 4 were comments.
Discussion
In this meta-analysis, we reviewed the association between HBV infection and risk of pancreatic cancer. The overall result reveals that HBsAg positive patients have an increased risk of developing pancreatic cancer. However, subgroup analysis showed that this markedly increased risk was largely due to the summary risk estimates from the studies of Asia region. we also summarized the impact of some other HBV infection states on the risk of pancreatic cancer, but the result reveals that past exposure to HBV with or without natural immunity does not associated with a risk of pancreatic cancer. These observations provided an evidence that HBV infection is a risk factor for pancreatic cancer. If the positive association between HBV infection and pancreatic cancer development is a true, so what mechanisms might explain this association? Anatomically, the proximity of the liver to the pancreas and share common blood vessels and ducts, meanwhile both the pancreas and liver are differentiated from interconnected endoderm cells, which makes the pancreas a potential target for hepatitis viruses [20].
From molecular perspective, HBV DNA in pancreatic duct epithelial cells of patients with pancreatic cancer complicated with HBV infection has been detected by immunohistochemistry and hybridization [8,21]. On the one hand, persistent HBV infection can cause persistent chronic inflammatory stimulation, which in turn changes the viscoelasticity of pancreatic tissue. The increased stiffness creates a mechanical cellular stress that produces excess oxygen free radicals through signaling pathways, which eventually leads to cancer [22]. On the other hand, HBV is a cancer-promoting virus, which can integrate HBV DNA into the genome of pancreatic cells, promote the occurrence and development of pancreatic cancer through modulation of the PI3K/AKT and JAK/STAT signaling pathways[23-24]. Therefore, the induction mechanism of HBV in hepatocellular carcinoma may also be applicable to pancreatic cancer. This meta-analysis is a secondary study, and its quality mainly depends on the quality of the original research, so it has some limitations. First, because nine of twelve studies [5-8,11- 15] used a case-control study, so the evidence intensity is relatively weak. Besides, the population included in this meta-analysis was mainly concentrated in Asia, so regional differences could affect the statistical results of the study.
Second, although we summarized the impact of some other HBV infection states on the risk of pancreatic cancer, but the results should be viewed with caution since some of the included studies had no information. In addition, only a few studies [8,9,16] have analyzed the correlation between content of HBV DNA and the risk of pancreatic cancer, so the risk assessment significance of this important indicator cannot be summarized and evaluated. Obviously, the number of included studies in this meta-analysis is still insufficient, and some included studies contains small sample size. Third, the meta-analysis itself is affected by uncontrollable confounders and publication bias, both of which can lead to differences in statistical results. In our meta-analysis, we adjusted for risk factors such as diabetes mellitus, smoking, drinking, chronic pancreatitis and body mass index, respectively. The results showed that HBV infection was positively correlated with the risk of pancreatic cancer after adjustment for diabetes mellitus, smoking, drinking and body mass index, but not after adjustment for chronic pancreatitis, suggesting that the residual confounding by chronic pancreatitis altered the association between HBV infection and the risk of pancreatic cancer. Finally, heterogeneity between studies is also a concern in the meta-analysis.
It is largely due to differences in race, study design and sample size and so on. The degree of heterogeneity was somewhat increased between the studies conducted in Asian, suggesting that race may be a potential source of heterogeneity. In conclusion, despite these limitations, our meta-analysis supports that HBV infection may increase the risk of pancreatic cancer. If the results are further confirmed by large numbers of population-based multicenter prospective studies, it will have an important clinical significance, which may narrow the scope of high-risk groups and improve the screening efficiency of pancreatic cancer. Meanwhile, primary prevention of pancreatic cancer is achieved through vaccination. Furthermore, the treatment of HBV infection should also be part of the comprehensive treatment of pancreatic cancer.
Conflict of Interests
The authors declare that there were no conflict of interest.
References
- Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics. CA Cancer J Clin 64: 9-29.
- Korc M, Jeon CY, Edderkaoui M, Pandol SJ, Petrov MS, et al (2017) Tobacco and alcohol as risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol 31: 529-536.
- Zheng Z, Zheng R, He Y, Sun X, Wang N, et al. (2016) Risk Factors for Pancreatic Cancer in China: A Multicenter Case-Control Study. J Epidemiol 26: 64-70.
- Thiruvengadam SS, Chuang J, Huang R, Girotra M, Park WG (2019) Chronic pancreatitis changes in high-risk individuals for pancreatic ductal adenocarcinoma. Gastrointest Endosc 89: 842-851.
- Hassan MM, Li D, El-Deeb AS, Wolff RA, Bondy ML, et al. (2008) Association between hepatitis B virus and pancreatic cance. J Clin Oncol 26: 4557-4562.
- Wang DS, Chen DL, Ren C, Wang ZQ, Qiu MZ, et al. (2012) ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int J Cancer 131: 461-468.
- Ben Q, Li Z, Liu C, Yuan Y, Wang K, et al. (2012) Hepatitis B virus status and risk of pancreatic ductal adenocarcinoma: a case-control study from China. Pancreas 41: 435-440.
- Jin Y, Gao H, Chen H, Wang J, Chen M, et al. (2013) Identification and impact of hepatitis B virus DNA and antigens in pancreatic cancer tissues and adjacent non-cancerous tissues. Cancer Lett 33:447-454.
- Iloeje UH, Yang HI, Jen CL, Su J, Wang LY, et al. (2010) Risk of pancreatic cancer in chronic hepatitis B virus infection: data from the REVEAL-HBV cohort study. Liver Int 30: 423-429.
- Desai R, Patel U, Sharma S, Singh S, Doshi S, et al. (2018) Association Between Hepatitis B Infection and Pancreatic Cancer: A Population-Based Analysis in the United States. Pancreas 47: 849-855.
- Hong SG, Kim JH, Lee YS, Young-Sun Lee, Eileen Yoon, et al. (2010) The relationship between hepatitis B virus infection and the incidence of pancreatic cancer: a retrospective case-control study. Korean J Hepatol 16: 49-56.
- Zhu F, Li HR, Du GN, Chen JH, Cai SR (2011) Chronic hepatitis B virus infection and pancreatic cancer: a case-control study in southern China. Asian Pac J Cancer Prev 12: 1405-1408.
- Woo SM, Joo J, Lee WJ, Sang-Jae Park, Sung-Sik Han, et al (2013) Risk of pancreatic cancer in relation to ABO blood group and hepatitis C virus infection in Korea: a case-control study.J Korean Med Sci 28: 247-251.
- Chang MC, Chen CH, Liang JD, Yu-Wen Tien, Chiun Hsu, et al. (2014) Hepatitis B and C viruses are not risks for pancreatic adenocarcinoma.World J Gastroenterol 20: 5060-5065.
- An J, Kim JW, Shim JH, Han S, Yu CS, et al. (2018) Chronic hepatitis B infection and non-hepatocellular cancers: A hospital registry-based, case-control study. PLoS One 13: e0193232.
- Huang J, Magnusson M, Törner A, Ye W, Duberg AS (2013) Risk of pancreatic cancer among individuals with hepatitis C or hepatitis B virus infection: a nationwide study in Sweden. Br J Cance 109: 2917-2923.
- Krull Abe S, Inoue M, Sawada N, Motoki Iwasaki, Taichi Shimazu, et al. (2016) Hepatitis B and C Virus Infection and Risk of Pancreatic Cancer: A Population-Based Cohort Study (JPHC Study Cohort II). Cancer Epidemiol Biomarkers Prev 25: 555-557.
- Andersen ES, Omland LH, Jepsen P, Krarup H5, Christensen PB, et al. (2015) Risk of all-type cancer, hepatocellular carcinoma, non-Hodgkin lymphoma and pancreatic cancer in patients infected with hepatitis B virus. J Viral Hepat 22: 828-834.
- Wells GA, Shea B, O’Connell D, J Peterson, V Welch, et al. (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Health Research Institute.
- Kamiza AB, Su FH, Wang WC, Sung FC, Chang SN, et al. (2016) Chronic hepatitis infection is associated with extrahepatic cancer development: a nationwide population-based study in Taiwan. Bmc Cancer 16: 861-869.
- Chen Y, Bai X, Zhang Q, Wen L, Su W, et al. (2016) The hepatitis B virus X protien promotes pancreatic cancer through modulation of the PI3K/AKT signaling pathway. Cancer Lett 380: 98-105.
- Fiorino S, Bacchi-Reggiani L, Pontoriero L, Claudio Gallo, Elisabetta Chili, et al. (2014) Tensegrity model hypothesis: may this paradigm be useful to explain hepatic and pancreatic carcinogenesis in patients with persistent hepatitis B or hepatitis C virus infection? JOP 15: 151-164.
- Chen Y, Bai X, Zhang Q, Wen L, Su W, et al. (2016) The hepatitis B virus X protein promotes pancreatic cancer through modulation of the PI3K/AKT signaling pathway. Cancer Lett 380: 98-105.
- Yang Y, Zheng B, Han Q,Cai Zhang, Zhigang Tian, et al. (2016) Targeting blockage of STAT3 inhibits hepatitis B virus-related hepatocellular carcinoma. Cancer Biology & Therapy 17: 449-456.

 Research Article
Research Article