Abstract
Recently, considerable attention has focused on the role of mitochondria in the maintenance of human health and the repercussions associated with mitochondrial dysfunction as they pertain to ageing, neurodegeneration and metabolic disease. Optimal mitochondrial homeostasis and function may diminish cellular oxidative stress and promote cellular health and longevity. Herein we investigate the cellular and mitochondrial effect of providing a synergistic blend of Tricarboxylic Acid Intermediates (pyruvate, malate, citrate and ascorbate) to immortalized C2C12 mouse myoblast cells to assess the value of nutritional intervention in this muscle cell model. These intermediates used in combination, as a mitochondrial-targeted health supplement (NPN80070757), are purported to offer a modality for mitochondrial augmentation therapy (MAT). In our hands, supplementation of cultured C2C12 myocyte cells using these intermediates significantly enhanced mitochondrial flux as demonstrated by lower lactate production, modulated intracellular mitochondrial re-organization, and influenced the levels of mitochondrial respiratory complex subunit proteins. Western blot assessment of representative respiratory chain and ATPase subunits demonstrated the greatest impact of nutritional supplementation on the protein abundance (37.7% increase) of cytochrome c oxidase (COX) subunit I, reflecting a proportional increase of the entire complex. Concurrent increases were noted in Succinate dehydrogenase subunit II, and the Core-protein of cytochrome c oxidoreductase (Complex III) in this myocyte cell culture model system.
Keywords: C2C12 myocytes; Differentiated Myocyte Culture; Nutritional Intervention; Mitochondria; Athletic Performance; Mitochondrial Augmentation Therapy; Recovery, Aerobic Metabolism; Krebs’s Cycle; Respiratory Chain; Cytochrome C Oxidase; Malic Acid; Citric Acid; Vitamin C; Pyruvic Acid; Supplements; Tricarboxylic Acid Cycle
Abbreviation: ATP: Aerobically Driven Cellular Adenosine Triphosphate; TCA: Tricarboxylic Acid Intermediates; MAT: Mitochondrial Augmentation Therapy
Introduction
Mitochondria represent the energy generation centre of the cell and are central to the production of aerobically driven cellular adenosine triphosphate (ATP), thereby providing energy equivalents to fuel cellular metabolism. In addition, supernumerary roles of these abundant cellular organelles are to maintain the cellular redox state [1], and represent important contributors of anaplerotic precursors [2,3], driving anabolic cellular processes [2]. Concordantly, oxidative metabolism represents the primary source of cell generated reactive oxidative species that can ultimately result in cellular oxidative stress and foster cellular aging [4] leading ultimately to organelle dysfunction. Collectively mitochondrial function, or rather dysfunction, contributes cellular aging, senescence, and metabolic disease [5-11], whereas optimal mitochondrial homeostasis and function may diminish cellular oxidative stress [12] and support mitochondrial biogenesis [13], while promoting cellular health and longevity [14-16].
In this report, we assessed the effect of supplementing C2C12 immortalized myocytes with tricarboxylic acid intermediates (TCA), a muscle derived cell line, chosen to model a tissue possessing a high metabolic demand and hence support and maintain a greater abundance of mitochondria. This cell line has been used as a model system to assess the impact of various interventions on mitochondrial function and biogenesis [17-24]. Herein, we provided a combination of key TCA intermediates, specifically pyruvate, citrate and malate, which supply carbon-subunits utilized by this aerobic metabolic pathway. Also included in the synergistic blend was ascorbic acid, which confers cellular and mitochondrial antioxidant properties [25, 26] and supports the biosynthesis of carnitine [27,28], an essential shuttling molecule for redistribution of carbon skeletons within the cells. The aim was to evaluate the value of on-market nutritional supplements that purport mitochondrial augmentation therapy (MAT) and support athletic performance and post-exercise recovery. Interestingly, pyruvate alone has been reported to induce mitochondrial biogenesis and influence mitochondrial mass, which resulted in augmented cellular respiration in pyruvate supplemented cells [24].The mouse myoblast C2C12 anchorage depended cells also enabled the assessment of extracellular lactate production, an indicator of mitochondrial substrate flux [20], and the determination of the abundance of mitochondria using quantitative fluorescence-immuno-histochemistry, and the specific level of mitochondrial-associated respiratory chain complex subunits by Western blot analysis.
Materials and Methods
C2C12 Cell Culture Conditions
C2C12, functionally equivalent to ATCC® CRL-1772™, were used for the in vitro culture studies essentially as described by Montgomery et al. [19]. Cells were grown in the D-MEM containing 4.5 g/L of glucose, 1% Pen/Strep, 1% glutamine, and 10% FBS and the flasks were incubated at 37⁰ C, in 5% CO2 atmosphere until a cellular confluence of 70-80% was achieved and then sub-cultured if cell fusion was not desired.
Supplementation Composition
The Supplemented condition was produced by adding a composition of TCA intermediates to seeded C2C12 cells and exposing the cells to the solution throughout the growth and differentiation culture period. One milliliter of a 500 x stock Supplement solution was diluted directly into 500 mL of D-MEM supplemented with 10% fetal calf serum to yield a final concentration of intermediates as follows:447.4 μM malic acid, 363.6 μM sodium pyruvate, 113.6 μM ascorbic acid and 182.9 μM sodium citrate. The composition and concentrations were established to be like an on-market nutritional supplement (NPN0070757) which targets mitochondria to enhance athletic performance and post-exercise recovery, while reducing delayed onset muscle soreness (DOMS).
Lactic Acid Determination in C2C12 Cultured Cells
Lactate production in C2C12 mouse myoblast cells was assessed after a 30-minute period of cellular glucose deprivation (starvation) to deplete intracellular glycogen reserves and reduce endogenous reserve substrates [29-32]. This treatment effectively challenges the cells in the absence of glucose and other exogenous nutrients supplied by the media. The media, from a confluent culture maintained in T25 flasks, was removed and cells were rinsed twice with 5 ml of PBS. After rinsing, the cells were incubated in 5 mL of PBS at 37⁰ C. Subsequently, a final concentration of 1 mM of each substrate(s) to be assessed, or combinations thereof, were added directly to the PBS solution and the cells were incubated for an additional 30 min at 37⁰ C. Following the second incubation, the solution was collected and used to directly to measure the lactate production levels, as described by Papanastasiou-Diamandi et al. [33]. All procedures were performed in triplicates unless otherwise noted.
Cell Preparation and Immunofluorescent labeling of Mitochondrial COXIV Subunits
Sterile glass coverslips were placed into 6 well plates either containing Supplemented media or non-supplemented controls. Identical cell numbers (4000 cells/mL) were seeded into each well and allowed to propagate for seven days. The media was changed every two to three days. Before the staining, the cells were fixed on the glass cover slips to preserve integrity and cellular structures, particularly nuclei and mitochondria. Media was removed and cells were washed with PBS and fixed with ice-cold 100% methanol for five minutes, and then washed with cold PBS 3 times five minutes each. Subsequently, the cells were blocked for 1 hour with a solution composed of 1% BSA prepared in 300 mM glycine and immune-reacted with an anti-COXIV antibody (Abcam; ab202554) in 1% BSA overnight at 5⁰C, which has been validated as a mitochondrial loading control. The antibody was diluted with 1% BSA to 1:1000 as directed by the manufacturer, and essentially as detailed by Donaldson [34]. The subsequent day, the cells were washed with PBS three times for five minutes each in blocking buffer, and immune-reacted with a commercially available secondary FITC-conjugate antibody diluted in 1% BSA/PBS solution (Abcam; ab6717). Representative visual fields contained least 40 cells/field to enable the quantification of the median fluorescent intensity (MFI) arising from the FITC signal (COX IV) and DAPI (nuclear) staining intensity. The DAPI MFI was used to normalize the background across the cells.
Western Blot Analysis
Western blot analysis was performed as described by Merante et al. [29] using a mouse total OXPHOS rodent monoclonal antibody cocktail specifically optimized to determine the abundance of respiratory chain complex and ATPase abundance in mouse mitochondria (Abcam; ab110413) which was specifically detected using a goat anti-mouse horse radishperoxidase labeled secondary antibody (Abcam; ab6789). The cocktail specifically detects: Complex I subunit NDUFB8 (30 kDa, not shown); Complex II subunit-B at 30 kDa; Complex III core protein-2 at 49.5 kDa; Cytochrome c oxidase subunit I at 40 kDa; and Complex V alphasubunit at 53 kDa. Imaging software was used for calculating the densitometic abundance of the resulting image bands. The densitometry value for the α-subunit of Complex V (ATPase) was used as an inter-well control to normalize the lane intensity and the ratio of Supplemented to control was used to establish the percentage change across the two conditions assessed.
Result
Lactate Production in Substrate Depleted Myocytes
Assessment of extracellular lactate effectively provides an indicator of substrate flux through the tricarboxylic acid cycle and will be elevated under conditions whereby the mitochondrial respiratory chain is impeded, or electron transfer is compromised [31]. Cellular lactate production can thereby be modulated by the availability of reducing equivalents in the form of NADH or FADH2, hence reflecting the cellular redox state [29,32]. The assessment of lactate production after a period of glucose deprivation suggests that particular substrates were rapidly assimilated by the mitochondria and were effectively used for energy production without the accumulation of lactate, indicating that flux through the mitochondrial tricarboxylic acid cycle is not impeded and progresses efficiently in this differentiated myocyte cell type.
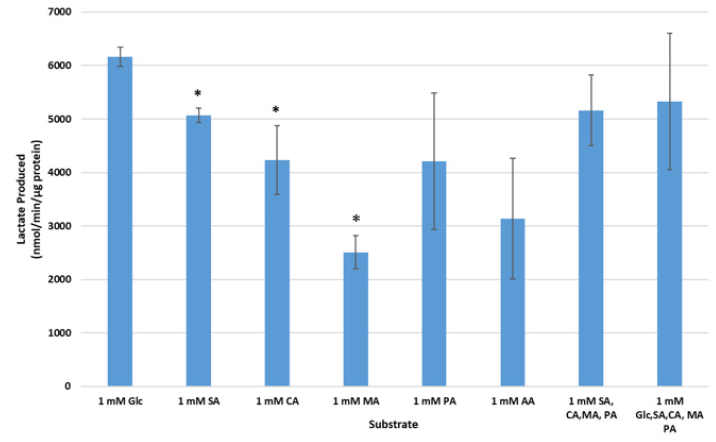
Figure 1 demonstrates that, as expected, relative to glucose, succinate, citrate and malic acid, when provided as single component additions, to the cells produced significantly lower extracellular lactate levels than glucose (p ≤ 0.05). Interestingly, the trend observed when pyruvic acid was directly supplied to myocytes, indicated that even with the increased pyruvate load, lactate production was not elevated. Rather, this critical substrate was readily taken up and used to fuel oxidative metabolism without transformation to lactate, suggesting enough flux capacity through the TCA cycle. In addition, the combination of substrates composed of succinic, citric, malic and pyruvic acids resulted in a noticeably, but not significant, lower extracellular lactate concentrations relative to when glucose was supplied as the sole carbon source. Finally, when glucose was supplied in the presence of tricarboxylic acid intermediates (succinic, citric malic and pyruvic acids), the trend was for lower lactate production relative to glucose alone for these combined substrates. Most notably, when glucose was the sole carbon source provided to the cells, post glycogen depletion, upon re-feeding, the confluent cell layer was noticeably negatively impacted and would peel from the plate. No such observation was noted when TCA cycle intermediates were used either in isolation or in combination either with or without glucose (data not shown).
Figure 1. Lactate production (nmol/min/μg protein) in substrate depleted cells. Extracellular lactate was assessed enzymatically after a 30 minute incubation in various TCA cycle substrates (n=3) post-substrate depletion, as follows: Succinic acid (SA), Citric acid (CA), Malic acid (MA), Pyruvic acid (PA), and Ascorbic acid (AA), or a combination thereof (n=3), relative to 1mM Glucose (Glc; n=2), (* p ≤ 0.05).
Immuno-fluorescent Assessment of Mitochondria Respiratory Chain and ATPase Complexes in Supplemented Cells
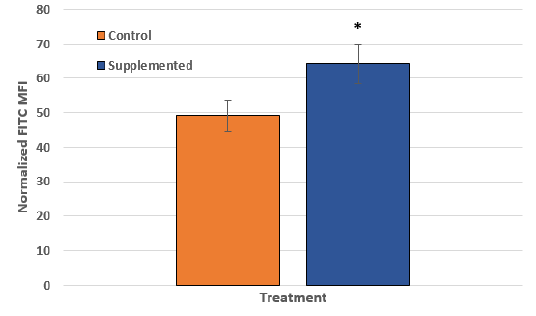
The assessment of mitochondria quantity, and possible intracellular re-organization of mitochondria, as a result of supplementation with TCA cycle intermediates was evaluated using immune-fluorescent FITC-labelling of a representative mitochondrial respiratory chain proteins comprising the cytochrome c oxidase complex, COXIV, and co-staining of nuclei with DAPI as a baseline control (Figure 2). This analysis determined that the median fluorescence intensity (MFI) of the COX IV (FITC signal), representative of the steady state respiratory chain subunit abundance obtained for un-supplemented (Control) cell mitochondria, was 49.11 ± 4.55 MFI, whereas in the Supplemented condition the signal intensity was significantly increased to 64.27 ± 5.76 MFI (p≤0.05), representing an approximate 20% increase relative to the control condition (Figure 3; n = 40). This result supported the hypothesis that there is a significant increase (p ≤ 0.05) in the steady state of COXIV abundance as reflected by the normalized ratio per field of view assessed, and hence the cytochrome c oxidase complex with supplementation. Also notable was a distinct reorganization of the mitochondria to the perinuclear region in Supplemented cells.
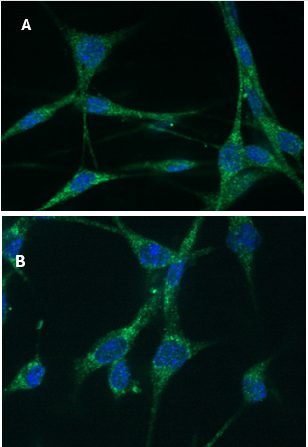
Figure 2. Immuno-fluorescent staining of Cytochrome c oxidase subunit IV (COXIV) and DAPI staining of nuclei in mouse C2C12 myoblasts. Cells were fixed onto glass cover slips and labelled by DAPI staining of the nucleus, and FITC staining of COX IV conjugated to the antibody targeting mitochondrial protein in the electron transport chain COX IV.
A) Mouse myoblast cells C2C12 grown in D-MEM control media.
B) Mouse C2C12 myoblast cells grown in Supplemented D-MEM media + 10% FBS. Median fluorescence intensity (MFI) of approximately 40 cells per field was determined across three fields of view across three slides.
Figure 3. The quantification of the intensity of FITC (mitochondrial) fluorescent signal (MFI) in C2C12 cells grown in unsupplemented (Control) and Supplemented D-MEM media. The DAPI (nuclear) signal was used as reference to normalize the FITC signal. The error bars represent the standard deviation of at least 40 measurements (cells) for each group across three fields per slide and three individual slides were assessed (* p ≤ 0.05).
Western Blot Analysis of Respiratory Chain and ATPase Subunits
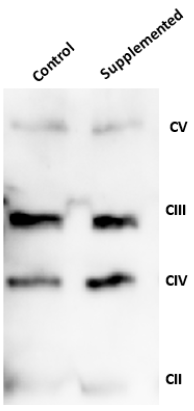
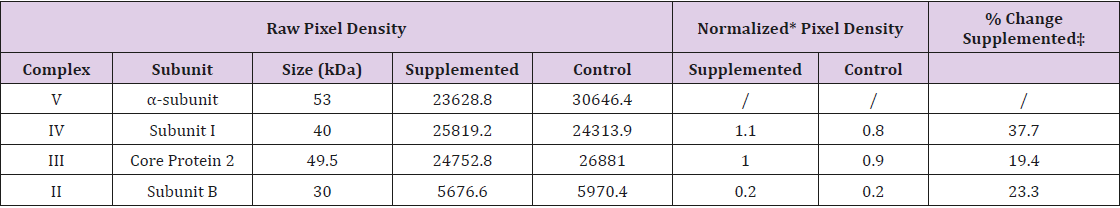
The relative abundance of various respiratory chain (Complexes I to IV) and ATPase (Complex V) protein subunits were simultaneously assessed in total cellular extracts by Western blot analysis using a commercially available antibody cocktail to compare among un-supplemented (Control) and Supplemented conditions (Figure 4). A demonstrable and reproducible change in protein abundance was noted for Cytochrome c oxidase subunit IV (COX IV) in Supplemented cultures whose abundance increased by approximately 37.7% indicating that nutritional intervention can influence mitochondrial protein abundance. Concurrently, the ATPase (Complex V, CV) (Figure 4) and Complex I (NDUFB8) remained unaffected Data not shown), while the succinate dehydrogenase (CII) and ubiquinol-cytochrome c oxidoreductase (CIII) subunits were increased by 20% relative to control cells (Figure 4 & Table 1).
Figure 4. Representative Western blot demonstrating the subunit abundance of mitochondrial electron transport chain complexes and the ATPase (CII-CV) in the mouse myoblasts cells C2C12 grown in Supplemented and control media. Total cellular proteins were extracted from differentiated C2C12 myoblast cells using Triton-X100 and 30 μg protein was assessed per well. Immuno-detection was performed using a commercial antibody cocktail (1:3000 dilution) targeting specific subunits of each of the complexes in the electron transport chain (Complex II, III and IV) and the ATPase (Complex V).
Table 1: Densitometry Analysis Summary of Respiratory Chain and ATPase subunit Abundance.
Note: * Pixel density normalized to the α-subunit of Complex V; ‡ Supplemented relative to control.
Discussion
The initial indication that supplementation cultured C2C12 myocytes with various TCA cycle intermediates influences substrate flux though the mitochondria was the noticeable and significant decrease in extracellular lactate levels. When glycogen depleted cells were provided glucose alone in the extracellular media, lactate production was significantly greater than when cells were provided an equal concentration of glucose supplemented with malate, pyruvate, citrate and succinate. Lactate production has been used diagnostically as a primary indicator of impediments (flux) to aerobic metabolism in multiple cell culture systems [31,32,35,36], and has been used to infer mitochondrial substrate flux [29,37-39]. These data suggest that in the Supplemented condition, mitochondrial flux is enhanced permitting rapid entry of the TCA intermediates into the TCA cycle and thereby minimizing the equilibrium-mediated conversion of pyruvate to lactate [36] prior to the entry into the TCA cycle.
To investigate if the provision of the combined TCA intermediates influenced mitochondrial quantity and/or protein content, cellular mitochondria was assessed via fluorescent immunostaining utilizing a fluorescein-labeled COX IV antibody to represent the steady state abundance of the subunits comprising this thirteen-subunit complex. Using COX IV for this purpose is particularly interesting as this subunit acts as a scaffolding protein and is necessary for the assembly of the COX complex [40]. Myoblast cells grown in media supplemented with a mix of intermediates (pyruvate, malate, citrate and ascorbic acid) had greater fluorescent intensity (MFI) arising from cytochrome c oxidase subunit IV staining relative to controls. Also, notable, but not quantified, was the distinct degree of perinuclear staining occurring in the supplemented cells supporting the notion that nutritional intervention may influence and modify mitochondrial positioning and anchoring [41]. The implications of this observation remain to be investigated. Quantitatively, the intensity of COX IV immune-detected mitochondrial protein content was approximately 25% greater in Supplemented cells, representing a significant increase in COX IV abundance. This observation was complemented by Western blot analysis using a cocktail of antibodies directed towards representative respiratory chain subunits and the mitochondrial ATPase (complex V). This assessment revealed that subunits representing Complex III (Coenzyme Q-cytochrome c oxidoreductase) and Complex II (Succinate dehydrogenase) were both elevated by 19.4 and 23.3%, respectively, in the supplemented condition. Complementing this observation was the significant increase in the steady state level of Complex IV Subunit-I, whose abundance was increased by 37.7%. This data supports, and is concordant with, the results obtained from immuno-fluorescent labelling of COX-IV and concurs with the effect of pyruvate supplementation on C2C12 cells reported by Wilson et al., [24].
The selection of TCA intermediates was dictated in part by their individual influences on mitochondrial function and muscle physiology, particularly as it relates to exercise and physical performance [42]. For example, malate supplementation has been shown to influence the expression of a number of antioxidant enzymes in rat liver mitochondria [43], improve aerobic capacity [44,45] and reduce oxidative stress [46]. Physiologically, oral administration of malate correlated with improved exercise performance and decreased muscle damage [45], while concomitantly increasing lifespan in model organisms [47]. Citrate supplementation alone has also been shown to influence athletic performance [48] and post-exercise recovery [49], while pyruvate supplementation may positively influence high-energy intermediates in neuronal tissue [44,50] and increase anaplerosis in skeletal muscle [51,52]. Finally, Ascorbic acid has been associated with redox modulation with the concomitant reduction of cellular oxidative stress [46], the fostering of myocyte cellular proliferation [53] and is actively recruited into active skeletal muscle to increase steady state levels in this tissue [27].
Collectively these data support a role for nutritional intervention in a complementary manner to that proposed by Damanti et al. [54] in myocyte associated tissues to help foster improved mitochondrial function, possibly by directing mitochondrial augmentation therapy using select TCA intermediates, in these and related cell types. These intermediates may collectively and synergistically influence mitochondrial dynamics, shape and nuclear DNA expression and epigenetics [25,55], illustrating the integrated role nutrient availability and metabolic flux can impart on cellular function and the modulation of mitochondrial biogenesis [13]. Acknowledgement We wish to thank and acknowledge Ms. Teresa Artuso for her critical reading of this document and excellent editing and constructive feedback. Many thanks to Ms. Tina Perricone for her project management excellence. We also acknowledge and thank the Ontario Centres of Excellence, Collaboration Voucher Program, for their continued support and the School of Biological Sciences and Applied Chemistry, chaired by Ms. Paola Battiston, at Seneca College for their commitment to enriched student training and engagement.
Conflict of Interest
The authors declare that there is no conflict of interest statement.
References
- Vall Llaura N, Mir N, Garrido L, Vived C, Cabiscol E (2019) Redox control of yeast Sir2 activity is involved in acetic acid resistance and longevity. Redox Biol pp. 101229.
- Anderson AJ, Jackson TD, Stroud DA, Stojanovski D (2019) Mitochondria hubs for regulating cellular biochemistry: emerging concepts and networks Open Biol 9(8): 190126.
- Walton ME, Ebert D, Haller RG (2003) Relative rates of anaplerotic flux in rested and contracted rat skeletal muscle measured by 13C NMR spectroscopy. J Physiol 548 (Pt 2): 541-548.
- Raha S, Robinson BH (2000) Mitochondria, oxygen free radicals, disease and ageing. TIBS 25(10): 502-508.
- Boengler K, Kosiol M, Mayr M, Schulz R, Rohrbach S (2017) Mitochondria and ageing: role in heart, skeletal muscle and adipose tissue. J Cachexia Sarcopenia Muscle 8(3): 349-369.
- Gray LR, Tompkins SC, Taylor EB (2014) Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci 71(14): 2577-2604.
- Jang JY, Blum A, Liu J, Finkel T (2018) The role of mitochondria in aging. J Clin Invest 128(9):3662-3670.
- Navarro A, Boveris A (2010) Brain Mitochondrial Dysfunction in Aging, Neurodegeneration, and Parkinson's Disease. Front Aging Neurosci 2: 34.
- Schiff M, Bénit P, Jacobs HT, Vockley J, Rustin P (2012) Therapies in inborn errors of oxidative metabolism. Trends Endocrinol Metab. 23(9): 488-495.
- Srivastava S (2017) The Mitochondrial Basis of Aging and Age-Related Disorders Genes (Basel) 8(12): 398.
- Sun N, Youle RJ, Finkel T (2016) The Mitochondrial Basis of Aging Mol Cell. Mol Cell 61(5): 654-666.
- Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, (2014) Oxidative Stress, Prooxidants, and Antioxidants: The Interplay Biomed Res Int 761264.
- Bouchez C,Devin A (2019) Mitochondrial Biogenesis and Mitochondrial Reactive Oxygen Species (ROS): A Complex Relationship Regulated by the cAMP/PKA Signaling Pathway 8(4): 287.
- Beach A, Leonov A, Arlia Ciommo A, Svistkova V, Lutchman V, et al. (2015) Mechanisms by Which Different Functional States of Mitochondria Define Yeast Longevity Int J Mol Sci 16(3): 5528-5554.
- Catic A (2018) Cellular Metabolism and Aging. Prog Mol Biol Transl Sci 155: 85-107.
- Hill S, Van Remmen H (2014) Mitochondrial stress signaling in longevity: A new role for mitochondrial function in aging. Redox Biol 2: 936-944.
- Bosutti A, Degens H (2015) The impact of resveratrol and hydrogen peroxide on muscle cell plasticity shows a dose-dependent interaction. Sci Rep 5: 8093.
- Le Borgne F, Ravaut G, Bernard A, Demarquoy J (2017) L-carnitine protects C2C12 cells against mitochondrial superoxide overproduction and cell death. World J Biol Chem 8(1): 86-94.
- Montgomery JL, Harper WM, Miller MF, Morrow KJ, Blanton JR (2002) Measurement of protein synthesis and degradation in C2C2) myoblasts using extracts of muscle from hormone treated bovine. Methods Cell Sci 24(4): 123-129.
- Nicholls DG, Darley Usmar VM, Wu M, Jensen PB, Rogers GW, et al. (2010) Bioenergetic profile experiment using C2C12 myoblast cells. J Vis Exp 6 (46): pii: 2511.
- Pronsato L, Milanesi L, Vasconsuelo A (2020) Testosterone induces up-regulation of mitochondrial gene expression in murine C2C12 skeletal muscle cells accompanied by an increase of nuclear respiratory factor-1 and its downstream effectors. MolCell Endocrinol 500: 110631.
- Schnuck JK, Gould LM, Parry HA, Johnson MA, Gannon NP, et al. (2018) Metabolic effects of physiological levels of caffeine in myotubes. J Physiol Biochem 74(1): 35-45.
- Vaughan RA, Garcia Smith R, Gannon NP, Bisoffi M, Trujillo KA, et al. (2013) Leucine treatment enhances oxidative capacity through complete carbohydrate oxidation and increased mitochondrial density in skeletal muscle cells. Amino Acids 45(4): 901-911.
- Wilson L, Yang Q, Szustakowski JD, Gullicksen PS, Halse R (2007) Pyruvate induces mitochondrial biogenesis by a PGC-1 alpha-independent mechanism. Am J Physiol Cell Physiol 292(5): C1599-605.
- Abdullah M, Jamil RT, Attia FN (2019) Vitamin C (Ascorbic Acid) 2019 Oct 21. StatPearls [Internet]. Treasure Island (FL): Stat Pearls Publishing.
- Sagun KC, Cárcamo JM, Golde DW (2005) Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J 19(12): 1657-1667.
- Carr AA, Bozonet SM, Pullar JM, Simcock JW, Vissers MCM (2013) Human skeletal muscle ascorbate is highly responsive to changes in vitamin C intake and plasma concentrations. Am J Clin Nutr 97(4): 800-807.
- Flanagan JL, Simmons PA, Vehige J, Willcox MDP, Qian G (2010) Role of carnitine in disease Nutr Metab (Lond) 7: 30.
- Merante F, Petrova Benedict R, Mac Kay N, Mitchell G, Lambert M, et al. (1993) A biochemically distinct form of cytochrome oxidase (COX) deficiency in the Saguenay-Lac-Saint-Jean region of Quebec. Am J Hum Genet 53(2): 481-487.
- Merante F, Mickle DA, Weisel RD, Li RK, Tumiati LC, et al. (1998) Myocardial aerobic metabolism is impaired in a cell culture model of cyanotic heart disease. Am J Physiol 275(5): H1673-681.
- Robinson BH, Mc Kay N, Goodyer P, Lancaster G (1985) Defective intramitochondrial NADH oxidation in skin fibroblasts from an infant with fatal neonatal lacticacidemia. Am J Hum Genet. 1985 37(5): 938-946.
- Robinson BH, Glerum DM, Chow W, Petrova Benedict R, Lightowlers R, et al. (1990) The use of skin fibroblast cultures in the detection of respiratory chain defects in patients with lacticacidemia. Pediatr Res 28(5): 549-555.
- Papanastasiou Diamandi A, Siskos PA, Diamandis EP (1983) A direct kinetic fluorimetric method for the enzymatic determination of lactate in plasma. Clin Chim Acta 129(3): 359-364.
- Donaldson JG (2015) Immunofluorescence Staining. Curr Protoc Cell Biol 69: 4.3.1-4.3.7.
- Debray FG, Mitchell GA, Allard P, Robinson BH, Hanley JA, et al. (2007) Diagnostic accuracy of blood lactate-to-pyruvate molar ratio in the differential diagnosis of congenital lactic acidosis. Clin Chem 53(5): 916-921.
- Robergs RA, Ghiasvand F, Parker D (2004) Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol 287(3): R502-516.
- Ly CH, Ryall JG (2017) Measuring Mitochondrial Substrate Utilization in Skeletal Muscle Stem Cells. Skeletal Muscle Development 61-73.
- Rao V, Merante F, Weisel RD, Shirai T, Ikonomidis JS, et al. (1998) Insulin stimulates pyruvate dehydrogenase and protects human ventricular cardiomyocytes from simulated ischemia. J Thorac Cardiovasc Surg. 116(3): 485-494.
- Robinson BH (1996) Use of fibroblast and lymphoblast cultures for detection of respiratory chain defects. Methods Enzymol 264: 454-464.
- Li Y, Park JS, Deng JH, Bai Y (2006) Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J Bioenerg Biomembr 38(5-6): 283-291.
- Kraft LM, Lackner LL (2018) Mitochondrial anchors: Positioning mitochondria and more. Biochem Biophys Res Commun 500(1): 2-8.
- Russell C, Papadopoulos E, Mezil Y, Wells GD, Plyley MJ, et al. (2014) Acute versus chronic supplementation of sodium citrate on 200 m performance in adolescent swimmers. J Int Soc Sports Nutr 11: 26.
- Zeng X, Wu J, Wu Q, Zhang J (2015) L-malate enhances the gene expression of carried proteins and antioxidant enzymes in liver of aged rats. Physiol Res 64(1): 71-78.
- Koivisto H, Leinonen H, Puurula M, Hafez HS, Barrera GA, et al. (2016) Chronic Pyruvate Supplementation Increases Exploratory Activity and Brain Energy Reserves in Young and Middle-Aged Mice. Front Aging Neurosci 8: 41.
- Wu JL, Wu QP, Huang JM, Chen R, Cai M, et al. (2007) Effects of L-malate on physical stamina and activities of enzymes related to the malate-aspartate shuttle in liver of mice. Physiol Res 56(2): 213-220.
- Wang N, Wei J, Liu Y, Pei D, Hu Q, et al. (2016) Discovery of biomarkers for oxidative stress based on cellular metabolomics. Biomarkers 21(5): 449-457.
- Edwards CB, Copes N, Brito AG, Canfield J, Bradshaw PC (2013) Malate and fumarate extend lifespan in Caenorhabditis elegans. PLoS One 8(3): e58345.
- Oöpik V, Saaremets I, Medijainen L, Karelson K, Janson T, et al. (2003) Effects of sodium citrate ingestion before exercise on endurance performance in well trained college runners. Br J Sports Med 37(6): 485-489.
- Timpmann S, Burk A, Medijainen L, Tamm M, Kreegipuu K, et al. (2012) Dietary sodium citrate supplementation enhances rehydration and recovery from rapid body mass loss in trained wrestlers. Appl Physiol Nutr Metab. 37(6): 1028-1037.
- Owen L, Sunram Lea SI (2011) Metabolic agents that enhance ATP can improve cognitive functioning: a review of the evidence for glucose, oxygen, pyruvate, creatine, and L-carnitine. Nutrients 3(8): 735-755.
- Constantin Teodosiu D, Peirce NS, Fox J, Greenhaff PL (2004) Muscle pyruvate availability can limit the flux, but not activation, of the pyruvate dehydrogenase complex during submaximal exercise in humans. J Physiol 561(Pt 2): 647-655.
- Constantin-Teodosiu D, Simpson EJ, Greenhaff PL (1999) The importance of pyruvate availability to PDC activation and anaplerosis in human skeletal muscle. Am J Physiol 276(3): E472-478.
- Duran BOS, Góes GA, Zanella BTT, Freire PP, Valente JS, et al. (2019) Ascorbic acid stimulates the in vitro myoblast proliferation and migration of pacu (Piaractus mesopotamicus). Sci Rep 9(1): 2229.
- Damanti S, Azzolino D, Roncaglione C, Arosio B, Rossi P, et al. (2019) Efficacy of Nutritional Interventions as Stand-Alone or Synergistic Treatments with Exercise for the Management of Sarcopenia. Nutrients 11(9).
- Bahat A, Gross A (2019) Mitochondrial plasticity in cell fate regulation J Biol Chem 294(38): 13852-13863.

 Research Article
Research Article