Commentary
It is generally agreed that age-related increases in arterial blood pressure (ABP) are mainly a reflection of an increase in systolic blood pressure (SBP) while maintaining or exhibiting a slight decrease in a diastolic blood pressure (DBP). This situation results in a widening of pulse pressure (PP) (i.e., the difference between SBP and DBP). It is generally thought that a widening of PP can be due to several different factors:
1. Valve regurgitation;
2. Aortic stiffening;
3. Severe iron deficiency; and/or
4. Hyperthyroidism [1,2].
Whatever the exact cause, this often leads to atrial fibrillation (AF) and is associated with coronary arterial disease or a heart attack. Often a change in diet and lifestyle changes can ameliorate the condition. If not, various medications are given to the patient such as angiotensin II converting enzyme inhibitors, beta-blockers, folic acid plus pyridoxine, and a variety of antihypertensive drugs. However, often these dogs have very little benefit and the situation is complicated by a developing atherogenesis [1-3].
Approximately 35 years ago, four of us found that under controlled laboratory conditions, using rats subjected to dietary deficiency of magnesium (Mg), that we observed a progressive elevation in ABP with a progressive widening of PP [4-6, unpublished findings]. Using high-resolution in-vivo TV imageintensification microscopy (up to 6,400 x normal), we found that there was a narrowing of arterioles, metarterioles , precapillary sphincters, and muscular venules concomitant with an increased vascular reactivity to a variety of vasoconstrictor agents ( e.g., norepinephrine, epinephrine, angiotensin II and several vasoactive peptides) in intact intestinal and skeletal muscle micro vessels [4-6]. Years later, other workers confirmed several of our findings [7,8]. We also found that aged rats showed severe distortion of the intact endothelial cells lining the arteriolar walls [[9, 10, 11], unpublished findings]. Feeding the rats diets low in Mg exacerbated the distortion of the intact endothelial cells; the older the animals, the greater the distortions [11]. These geometric changes were highly correlated to the changes in PP (P<0.001) [11].
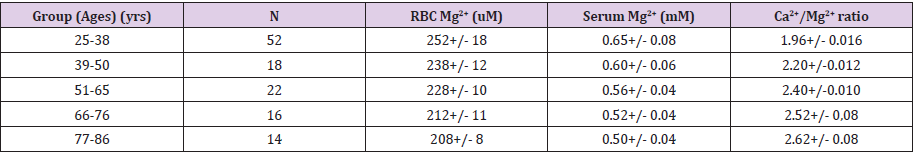
Our group and that of others have reported, in several studies, that aging human subjects (from infants to the elderly) demonstrate a progressive decline in both total and ionized serum Mg ( [Mg2+]o); with the ionized fraction showing the greatest progressive decline; the older the test subject , the lower the [Mg2+]0 [12-18]. The ionized fraction is the most significant as it parallels that of many tissue and cellular levels of free ionized Mg [19]. Those subjects with either type 2 diabetes mellitus, coronary artery disease, on renal dialysis, or hypertension demonstrated the greatest decline in [Mg2+]0, in serum and red blood cells, with age (e.g., see Table 1). Previously, in multiple human and animal studies, we documented that serum ionized Ca/Mg ratios are indicative of peripheral vasoconstriction and increased vascular reactivity [9, 10]. The results in Table 1 clearly demonstrate that as PP rises with age, so does the sera ionized Ca2+/Mg2+ ratios, suggesting that PP rises in the elderly reflect alterations in divalent cation concentrations.
Table 1: Serum and Red Blood Cell Ionized Mg Levels Decline with Age in Human Subjects Whereas Serum Ca2+/Mg2+ Ratios Rise.
Note:
1. Values are means +/- S.E.M. All values with each age group are significantly diff from one another (ANOVA, P<0.05).
2. RBC ionized Mg2+ were measured with 31P-NMR spectroscopy whereas the serum ionized Mg2+ and Ca2+ were measured with in-selective electrodes [12,19].
Decreased Dietary Intake of Mg Found in North American and European Populations and Risks for Cardiovascular Diseases and Strokes
At the turn of the last century, the North American and European populations were ingesting about 450-550 mg of Mg/ day [for recent reviews, see 20, 21]. Today, about 70-75% of these populations are ingesting only about 165-235 mg of Mg/ day [20, 21]. Many drinking waters contain only 5-10 mg of Mg/l. It has been shown, in multiple studies, that people drinking softwater (e.g., <10 mg/l of Mg) demonstrate a high risk for coronary arterial diseases, ischemic heart disease, hypertension, and strokes, whereas those peoples drinking hard—waters have low risks for these cardiovascular diseases and strokes [20,21].
Evidence for Progressive Release of Ceramides and Platelet-Activating Factor with Widening of Pulse Pressure in Experimental Mg Deficiency
About 25 years ago, working with isolated cerebral vascular and aortic smooth muscle cells, and 1H-nuclear magnetic resonance spectroscopy, our group found that decreased extracellular Mg resulted in an increase in sphingolipids, particularly ceramides, increases in platelet-activating factor (PAF) and PAF-like lipids [22,23]. All three of these classes of molecules can cause constriction/contraction of microvascular arterioles and venules as well as cerebral and coronary blood vessels [24-26].
Approximately 20 years ago, using rats placed on Mg deficient diets for 21 days, we noted an increased release of ceramides and platelet-activating factor (PAF) into the blood stream [26, 27, unpublished findings]. Some years later, we found that cardiovascular tissues and cells excised from these Mg-deficient animals demonstrated increased levels of ceramides; the greater the deficit in extracellular and intracellular free Mg ([Mg2+]), the greater the cellular rise in ceramides [26-30]. A few years later, we noticed that when primary cultured vascular smooth muscle cells (i.e., piglet coronary, cerebral vascular and aortic smooth muscle cells) were exposed to media with low Mg2+, there was a rapid rise in cellular PAF levels which could be inhibited with specific PAF receptor blockers; the longer the primary cells were kept in low [Mg2+ ]0 , up to a point, the greater the release of PAF-like lipids [31]. In view of such new experiments, we went-on to determine if the rises in PP in Mg-deficient animals were correlated positively with incremental rises in ceramides and PAF. We were not surprised that the answer was “yes” and that these rises in ceramides, PAF and PP could be significantly ameliorated with inhibitors of ceramide and PAF synthesis (P<0.01) [31].
Progressive Decrease of Ionized Magnesium Results in a Progressive Downregulation of Telomerase
Working with rats fed low Mg diets for 21 days, we found that cardiovascular tissues and cells demonstrated a downregulation of telomerases and oxidation of DNA [32]. Using specific ELISA assays, we found that the extirpated cardiovascular tissues and cells showed that, as the ionized Mg fell in both the blood and cardiovascular tissues, there was a greater and greater downregulation of telomerases and oxidation of DNA [31]. As we have previously suggested [32], these studies would be compatible with the idea that even short-term Mg deficiency in the aging process could cause alterations in the genome that would result in atherogenesis and increases in PP in the elderly.
Conclusions and Future Thoughts
Why there is a widening of PP in the aged has brought forth numerous hypotheses. More than 30 years ago, our laboratories noted that Mg- deficient animals subjected to low Mg diets, demonstrated a widening of PP with age and length of time on Mg-deficient diets. Since the North American and European populations are now known, for the most part, to be deficient in Mg intake, which appears to be correlated to rises in arterial blood pressure, coronary arterial diseases, ischemic heart disease, atherosclerosis, and strokes, we believe that our results, summarized above, implicating synthesis and releases of ceramides and PAF, as well as a downregulation of telomerases with rises in PP, in Mg-deficient animals, may be very significant. In view of our findings, we believe it will be very important to determine, in the aged population, whether the levels of Mg2+ are correlated to the rises in blood and tissue levels of ceramides and PAF and downregulation of telomerases. If the latter turns out to be correct, inhibitors of ceramide and PAF synthesis and release should be employed to determine if they will ameliorate the rises in PP, in the aged populations, in North America and Europe.
Acknowledgements
Many of our studies were supported, in part, by research grants from several divisions of The National Institutes of Health (i.e., The National Heart, Lung and Blood Institute; The National Institute on Drug Abuse; The National Institute of Mental Health; and The National Institute on Alcoholism and Alcohol Abuse} awarded to BMA and BTA. Several pharmaceutical companies gave us unrestricted grants-in-aid (i.e., The UpJohn Co., Sandoz Pharmaceuticals; CIBA-GEIGY Corp.; The Bayer Co.; and Pfizer Pharmaceuticals). While our original investigative studies were ongoing, Professor Lawrence M. Resnick and Forensic scientist Anthony Carella, unfortunately, both passed away. Both eminent scientists will be sorely missed.
References
- Russo D, Morrone LF, Brancaccio S, Napolitano P, Salvatore E, et al. (2009) Pulse pressure and presence of coronary artery calcification. Clin J Am Soc Nephrol 4(2): 316-322.
- Steppan J, Barodka V, Berkowitz DL, Nyhan D (2011) Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol Res and Practice.
- Laurent S, Tropeano AI, Boutouyrie P (2006) Pulse pressure reduction and cardiovascular protection. J Hypertens Suppl 24(3): S13-S18. https://doi.org/10.21694/1575-7601.17005
- Altura BM, Altura BT, Gebrewold A, Ising H, Gunther T, et al. (1984) Magnesium deficiency and hypertension: correlation between magnesium deficiency diet and microcirculatory changes in situ. Science 223(4642): 1315-1317.
- Altura BM, Altura BT, Gebrewold A, Gunther T, Ising H, et al. (1992) Noise-induced hypertension and magnesium in rats: relationship to microcirculation and calcium. J Appl Physiol 72(1): 194-202.
- Altura BM, Gebrewold A, Carella A, Shah NC, Altura BT, et al. (2018) Exposure to high levels of noise poses risks for development of hypertension and heart disease: Potential roles of unrecognized ionized magnesium deficiency and release of ceramides and platelet-activating factor. Am Res J Cardiovasc Dis 2(1).
- Laurant P, Hayoz D, Brunner HR, Berthelot A (1999) Effect of magnesium deficiency on blood pressure and mechanical properties of rat carotid artery. Hypertens 33(5): 1105-1110.
- Luthringer C, Raysisiguier Y, Gueux E, Berthelot A (1988) Effect of magnesium deficiency on serum lipids, blood pressure and cardiovascular reactivity. Br J Nutr 59(2): 243-250.
- Altura BM, Altura BT (1995) Magnesium and cardiovascular biology and atherogenesis. Cell Mol Biol Res 41(5): 347-360.
- Altura BM, Altura BT (2007) Magnesium: forgotten mineral in cardiovascular biology and atherogenesis. In: New Perspectives in Magnesium Research 239-260.
- Altura BM, Gebrewold A, Altura BT (2019) Magnesium deficiency results in widening of pulse pressure increased vascular reactivity and distortions in microvascular smooth muscle and endothelial cells in aging rats: An in-vivo microcirculatory study.
- Altura BT, Altura BM (1991) Measurement of ionized magnesium in whole blood, plasma and serum with a new ion-selective electrode in healthy and diseased subjects. Magnesium and Trace Elem 10(2-4): 90-98.
- Markell MS, Altura BT, Sarn Y, Delano BG, Ifudu Q, et al. (1993) Deficiency of ionized magnesium in patients receiving hemodialysis or peritoneal dialysis. ASAIO J 39(3): M801-M804.
- Marcus JC, Valencia JB, Altura BT, Cracco RQ, Jean-Baptiste D, et al (1998) Serum ionized magnesium in premature and term infants. Pediatr Neurol 18(4): 311-314.
- Munoz R, Wessel DR (2000) Whole blood ionized magnesium: age-related differences in normal values and clinical implications of ionized hypomagnesemia in patients undergoing surgery for congenital cardiac diseases. J Thor and Cardiovasc Surg 119(5): 891-899.
- Barbagallo M, Belvedere M, Dominguez LJ (2009) Magnesium metabolism and aging. Magnesium Res 22(4): 235-246.
- Barbagallo M, Di Bella G, Brucato V, D’Angelo D, Damiani D, et al. (2014) Serum ionized magnesium in older persons. Metabol 63(4): 502-509.
- Altura BM, Li W, Zhang A, Zheng T, Shah NC, et al. (2016) Sudden cardiac death in infants, children and young adults: possible roles of dietary magnesium intake and generation of platelet-activating factor in coronary arteries. J Heart Health 2(2). http://dx.doi.org/10/16966/2379-769X.121
- Altura BM, Altura BT (2016) Importance of ionized magnesium measurements in physiology and medicine and the need for ion-selective electrodes. J Clin Case Stud 1(2).
- Dean C (2014) The Magnesium Miracle. Ballantine Books, New York.
- Altura BM, Zhang A, Murakawa T, Zheng T, Li W, et al. (2019) Can hypomagnesemia put the squeeze on coronary arteries: An unappreciated factor in myocardial ischemia, heart attacks and sudden cardiac death. EC Orthop 10(7): 572-581.
- Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, et al. (1997) Mg2+ modulates membrane lipids in vascular smooth muscle: a link to atherogenesis. FEBS Lett 408(2): 191-194.
- Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, et al. (1998) Mg2+ modulates membrane sphingolipid and lipid second messengers in vascular smooth muscle cells. FEBS Lett 440(1-2): 167-171.
- Altura BM, Gebrewold A, Zheng T, Altura BT (2002) Sphingomyelinase and ceramide analogs induce vasoconstriction and leukocyte-endothelial interactions in cerebral venules in the intact rat brain: Insight into mechanisms possible relation to brain injury and stroke. Brain Res Bull 58(3): 271-278.
- Zheng T, Li W, Wang J, Altura BT, Altura BM, et al. (2000) Sphingomyelinase and ceramide analogs induce contraction and rapid rises in [Ca2+]I in canine cerebral vascular muscle. Am J Physiol Heart Circ Physiol 278(5): H1421-H1428.
- Altura BM, Shah NC, Shah GJ, Altura BT (2018) Magnesium deficiency sphingolipids and telomerase: Relevance to atherogenesis, cardiovascular diseases and aging. In: Handbook of Starvation, Famine and Nutrient Deprivation, Preedy V, Patel V, eds. , Springer Nature, Berlin.
- Altura BM, Shah NC, Li Z, Jiang X-C, Perex-Albela JL, et al. (2010) Magnesium deficiency upregulates serine palmitoyltransferase (SPT1 and SPT2) in cardiovascular tissues : relationship to serum ionized Mg and cytochrome C. Am J Physiol Heart Circ Physiol 299(3): H932-H938.
- Altura BM, Shah NC, Li Z, Jiang X-C, Zhang A, et al. (2010) Short-term magnesium deficiency upregulates sphingomyelinase synthase and p53 in cardiovascular tissues and cells: Relevance to the de novo synthesis of ceramide. Am J Physiol Heart Circ Physiol 299: H2048-H2055.
- Altura BM, Shah NC, Shah GJ, Zheng T, Li W, et al. (2012) Short-term magnesium deficiency upregulates ceramide synthase in cardiovascular tissues and cells: crosstalk among cytokines, NF-kB and de novo ceramide. Am J Physiol Heart Circ Physiol 302(1): H319-H332.
- Altura BM, Shah NC, Shah GJ, Li W, Zhang A, et al. (2013) Magnesium deficiency upregulates sphingomyelinases in cardiovascular tissues and cells: crosstalk among proto-oncogenes, Mg2+, NF-kB and ceramide and their potential relationships to resistant hypertension, atherogenesis and cardiac failure. Int J Clin Exp Med 6(10): 861-869.
- Altura BM, Li W, Zhang A, Shah NC, Shah GJ, et al. (2016) The expression of platelet-activating factor is induced by low extracellular Mg in aortic, cerebral and neonatal coronary vascular smooth muscle ; cross-talk with ceramide production, NF-kB and proto-oncogenes: possible links to atherogenesis and sudden cardiac death in children and infants, and aging: Hypothesis, review and viewpoint. Int J Cardiol Res 3(1): 47-67.
- Shah NC, Shah GJ, Li Z, Jiang XC, Altura BT, et al. (2014) Short-term magnesium deficiency downregulates telomerase , upregulates sphingomyelinase and induces DNA damage in cardiovascular tissues: relevance to atherogenesis, cardiovascular diseases and aging. Int J Clin Exp Med 7(3): 497-514.

 Commentary
Commentary